��Ŀ����
�����£�Ũ�Ⱦ�Ϊ0.1 mol/L��������Һ��
��Na2CO3��Һ NaHCO3��Һ ������ �ܰ�ˮ
�Իش��������⣺
��1��������Һ����ˮ�ⷴӦ�������� �����ţ���ͬ������Һ�����ʴ��ڵ���ƽ����� ��
��2���ȽϢ١�����Һ��PHֵ�ϴ���� ��
��3������Һ���м�������NH4Cl���壬��ʱ![]() ��ֵ�� �����С����������䡱����
��ֵ�� �����С����������䡱����
��4������Һ�۵ζ�V mL��Һ�ܣ���ζ���������ͼ��ʾ��
���Է����ڵζ������У��ζ�������a��b��c��d�ĵ㣺
ˮ�ĵ���̶������� �㣬�����ǣ�
��
��a����Һ������Ũ�ȴ�С��ϵ�� ��
��ȡ����c����Һ���Թܣ��ٵμ�0.1 mol/L NaOH��Һ�����ԡ���ʱ��Һ�г�H����OH���⣬����Ũ�ȴ�С��ϵ�� ��
��1���٢ڣ�1�֣��ڢܣ�1�֣�
��2���٣�1�֣�
��3�����1�֣�
��4����c��1�֣�����Һǡ�÷�Ӧ����NH4Cl����NH4��ˮ��ٽ�ˮ�ĵ��룬���������ʾ����ʣ�������ˮ�ĵ��룬��c��ˮ�ĵ���̶����2�֣�
��c��NH4������c��Cl������c��OH������c��H������1�֣�
��c��Cl������c��NH4������c��Na������2�֣�
����:

 Ʒѧ˫�ž�ϵ�д�
Ʒѧ˫�ž�ϵ�д� Сѧ��ĩ���100��ϵ�д�
Сѧ��ĩ���100��ϵ�д�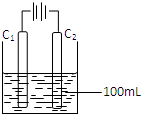 ���û�ѧ֪ʶ��������������е��й����ⱸ�ܹ�ע����ش��������⣺
���û�ѧ֪ʶ��������������е��й����ⱸ�ܹ�ע����ش��������⣺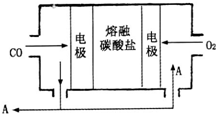 ��2013?����ģ�⣩�Ƽ��仯������й㷺����;��
��2013?����ģ�⣩�Ƽ��仯������й㷺����;��