��Ŀ����
ûʳ����������PG���ṹ��ʽΪ  ���ǰ�ɫ��ĩ��������ˮ�����������͵���֬���dz��õ�ʳ���Ϳ���������
���ǰ�ɫ��ĩ��������ˮ�����������͵���֬���dz��õ�ʳ���Ϳ���������
��1��PG�ķ���ʽΪ ����д��PG���������������ŵ����� ��1molPG����������������Һ��ȫ��Ӧʱ�����ĵ��������Ƶ����ʵ����� ��
PG�ɷ�������ת����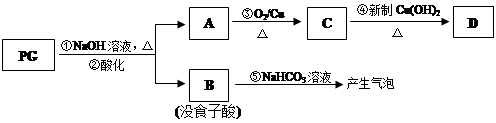
��2��A�Ľṹ��ʽΪ ��1molûʳ���������� mol H2�ӳɡ�
��3����ͼ���йر仯�У�����������Ӧ���У�����ţ� ��
��4���ӷ��ӽṹ�������Ͽ���PG���п��������õ���Ҫԭ���ǣ�����ţ� ��
a�����б��� b�������Ȼ� c�����з��ǻ� d.����ʳ����
��5����Ӧ�ܵĻ�ѧ����ʽΪ��
��6��B�ж���ͬ���칹�壬д�����з�������Ҫ���ͬ���칹��Ľṹ��ʽ��
i�����б������ұ����ϵ�һ�����ֻ��һ�֣�ii�����ܷ���������Ӧ�����ܷ���ˮ�ⷴӦ��
��1��C10H12O5���ǻ���������4mol����1�֣�
��2��CH3CH2CH2OH��1�֣��� 3��1�֣���
��3���ۢ� ��2�֡���һ����1�֣���һ����1�֡���
��4��c��1�֣�
��5��CH3CH2CHO �� 2Cu(OH)2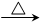 CH3CH2COOH��Cu2O����2H2O��2�֣�����ƽ�ĸ�1�֣�
CH3CH2COOH��Cu2O����2H2O��2�֣�����ƽ�ĸ�1�֣�
��6��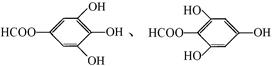 ����1�֣�
����1�֣�
���������������1���������֪��PG�ķ���ʽΪC10H12O5��PG����������Ϊ�ǻ���������1molPG��ˮ��ȫ��Ӧ���������к���3mol���ǻ���1mol�Ȼ���1mol���ǻ������ǻ������ԣ�����������4molNaOH����2����ת����ϵ��֪��AΪ������CH3CH2CH2OH����1molûʳ�����еı�������3molH2�����ӳɷ�Ӧ����3��ͼ�Т٢ڢۢܢݷֱ���ȡ����Ӧ��ȡ����Ӧ��������Ӧ��������Ӧ��ȡ����Ӧ�ֽⷴӦ����4���������ױ������е�����������PG���ж�����ǻ������к�ǿ�Ļ�ԭ�ԣ���˿�����������������c��ȷ����5��C��D�ֱ�Ϊ��ȩ�����������ƣ���Ӧ��ΪCH3CH2CHO��2Cu(OH)2 CH3CH2COOH��Cu2O����2H2O��CH3CH2CHO�� 2Cu(OH)2+NaOH
CH3CH2COOH��Cu2O����2H2O��CH3CH2CHO�� 2Cu(OH)2+NaOH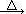 CH3CH2COONa��Cu2O����3H2O����6���������֪����ͬ���칹�����ڼ���ij����������1���ǻ��������������λ�ã�����2���ǻ�λ���������ǻ�������Գ�λ�á�
CH3CH2COONa��Cu2O����3H2O����6���������֪����ͬ���칹�����ڼ���ij����������1���ǻ��������������λ�ã�����2���ǻ�λ���������ǻ�������Գ�λ�á�
���㣺�����л��ϳɺ��л��ƶϵ����֪ʶ��

��������Ҫ�Ĺ�ҵԭ��֮һ����ҵ�ϲ��ñ��Ӻϳ��л���D��·�����£�
��ش��������⣺
(1)�л���A���ӹ������� (д����)��B�Ľṹ��ʽ�� ����˴Ź�������ͼ���� �ַ塣
(2)C��D�ķ�Ӧ������ ��D������NaOH��Һ����ʱ��Ӧ�Ļ�ѧ����ʽ��
(3)X��B��ͬ���칹�壬X�����к��б��������б�����һ�ȴ���ֻ�����֣����ܷ���������Ӧ����X�����нṹ��ʽ�� �� �� �� ��
�� �� �� ��
(4)�йػ�����C��˵����ȷ���� (����ĸ)
| A���ܷ����ӳɷ�Ӧ |
| B��һ�������£�������NaOH����Һ�з�����ȥ��Ӧ |
| C��1molC������NaOH��Һ���ȣ��������2molNaOH |
| D�������������� |








 ������ͨ����ͬ��ѧ��Ӧ�ֱ��Ƶ�B��C��D�������ʣ�
������ͨ����ͬ��ѧ��Ӧ�ֱ��Ƶ�B��C��D�������ʣ�