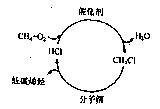
��Ŀ����
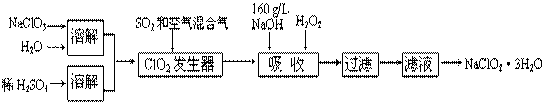
��10�֣��������ƣ�NaClO2����һ����Ҫ�ĺ����������������ǹ������ⷨ�����������ƵĹ�������ͼ
��֪����NaClO2���ܽ�����¶����߶������ʵ������¿ɽᾧ����NaClO2��3H2O��
��ClO2�ķе�Ϊ283 K����ClO2�ֽⱬը������ϡ����������ϡ�ͷ�ֹ��ը�Էֽ�
��HClO2��25 ��ʱ�ĵ��볣��������ĵڶ������볣���൱������Ϊǿ�ᡣ
��1��160 g/L NaOH��Һ�����ʵ���Ũ��Ϊ____________________ ����Ҫ�������Һ����������������Ҫ��һ��������__________________ ��
��2��ClO2����������������Ӧ�����ӷ���ʽΪ__________________________
��3����������Ϊ��ֹ����NaClO2��������ԭ��NaCl�����û�ԭ���Ļ�ԭ��Ӧ���С���H2O2����������ѡ��Ļ�ԭ���ǣ� ��������ţ�
��4��д���������з�����Ӧ�Ļ�ѧ����ʽ____________________________
��5������Һ�еõ�NaClO2��3H2O���廹������еIJ�����_____________ ����������裩

��֪����NaClO2���ܽ�����¶����߶������ʵ������¿ɽᾧ����NaClO2��3H2O��
��ClO2�ķе�Ϊ283 K����ClO2�ֽⱬը������ϡ����������ϡ�ͷ�ֹ��ը�Էֽ�
��HClO2��25 ��ʱ�ĵ��볣��������ĵڶ������볣���൱������Ϊǿ�ᡣ
��1��160 g/L NaOH��Һ�����ʵ���Ũ��Ϊ____________________ ����Ҫ�������Һ����������������Ҫ��һ��������__________________ ��
��2��ClO2����������������Ӧ�����ӷ���ʽΪ__________________________
��3����������Ϊ��ֹ����NaClO2��������ԭ��NaCl�����û�ԭ���Ļ�ԭ��Ӧ���С���H2O2����������ѡ��Ļ�ԭ���ǣ� ��������ţ�
| A��Na2O2 | B��Na2S | C��FeCl2 | D��KMnO4 |
��5������Һ�еõ�NaClO2��3H2O���廹������еIJ�����_____________ ����������裩
��10�֣���1��4mol/L ��Һ���ܶ�
��2��2ClO3��+SO2=SO42-+2ClO2
��3�� A ��4��2ClO2+H2O2+2NaOH=2NaClO2+O2+2H2O
��5���ؽᾧ
��2��2ClO3��+SO2=SO42-+2ClO2
��3�� A ��4��2ClO2+H2O2+2NaOH=2NaClO2+O2+2H2O
��5���ؽᾧ
��

��ϰ��ϵ�д�
�����Ŀ
 2S�ɲ��ô���Ǵ�ת����������CH4ת����CO���õ�CO��CO2��H2�Ļ�����壬������ĺϳɼ״�ԭ���������ɽ��м״��ϳɡ�
2S�ɲ��ô���Ǵ�ת����������CH4ת����CO���õ�CO��CO2��H2�Ļ�����壬������ĺϳɼ״�ԭ���������ɽ��м״��ϳɡ�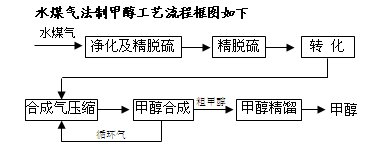
 CO��g��+H2��g�����˷�Ӧ�����ȷ�Ӧ���ٴ˷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪ ��
CO��g��+H2��g�����˷�Ӧ�����ȷ�Ӧ���ٴ˷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪ �� ��ʩ�� ��(����ĸ����)
��ʩ�� ��(����ĸ����) CH3OH(g)����H ����90.8kJ��mol��1��T4���´˷�Ӧ��ƽ�ⳣ��Ϊ160�����¶��£����ܱ������м���CO��H2����Ӧ��ijʱ�̲�ø���ֵ�Ũ�����£�
CH3OH(g)����H ����90.8kJ��mol��1��T4���´˷�Ӧ��ƽ�ⳣ��Ϊ160�����¶��£����ܱ������м���CO��H2����Ӧ��ijʱ�̲�ø���ֵ�Ũ�����£� ��
��