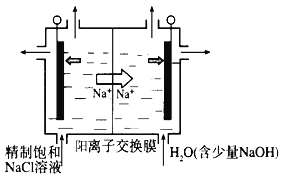
��Ŀ����
����Ŀ�����ᱵ�����ǵ����մ�Ԫ��������Ҫ����ԭ�ϣ����Ʊ���������ͼ���£�
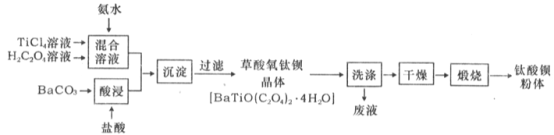
(l)������̼Ԫ�صĻ��ϼ�Ϊ_____��
(2)Ϊ���BaCO3��������ʣ��ɲ�ȡ�Ĵ�ʩΪ_____ ��д��һ������
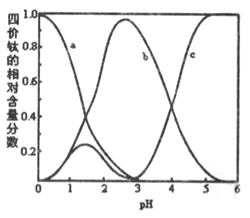
(3)�������Һ�����ڣ���Ԫ���ڲ�ͬpH����Ҫ��TiOC2O4��TiO(C2O4)22-����TiO(OH)+������ʽ���ڣ���ͼ��ʾ��ʵ���Ʊ������У����ð�ˮ���ڻ����Һ��pH��2.5��3֮�䣬�ٽ��С�������������ͼ������a��Ӧ�ѵ���ʽΪ________���ѧʽ����д����������ʱ�����ӷ���ʽ��__________��
(4)�������ѱ����徭����ˮ����ϴ�Ӻ�֤�����ѱ�ϴ�Ӹɾ���ʵ�鷽��____________��
(5)��ҵ��TiCl4��BaCO3ͨ�������·����Ʊ���
���Ƚ����ʯ(TiO2)�������̿��ϣ���ͨ��Cl2�����ȵ�900����ȡTiCl4����д���÷���ȡTiCl4�Ļ�ѧ����ʽ��__________________________________��
��BaCO3�ǽ��ؾ�ʯ����Ҫ�ɷ�ΪBaSO4��������Na2CO3��Һ���㹻��ʱ�����Ʊ�������Na2CO3��Һ��Ũ������Ҫ����______ mol��L-1���ܿ�ʼת����(��֪�����£�Ksp(BaSO4)=1.0��10-10(mol��L-1)2��Ksp(BaCO3) =2.58��10-9(mol��L-1)2������CO32-��ˮ�⣩��
���𰸡� +3 ��BaCO3�гɷ�ĩ���ʵ���������Ũ�ȡ��ʵ����ȡ����裨��дһ���� TiOC2O4 TiO(C2O4)22-+ Ba2+ + 4H2O = BaTiO(C2O4)2��4H2O�� ȡ���һ��ϴ��Һ�������Թ��У������еμ�AgNO3��Һ���ް�ɫ��������֤���������ѱ��ѱ�ϴ�Ӹɾ� TiO2+2C+2Cl2![]() TiCl4+2CO 2.58��10-4
TiCl4+2CO 2.58��10-4
����������1����H2C2O4��H��C��O�Ļ��ϼ۷ֱ�Ϊ+1��+3��-2����2��������BaCO3�гɷ�ĩ���ʵ�����Ӧ�������Ũ�ȡ��ʵ����ȡ����ٽ��趼���Լӿ췴Ӧ���ʣ���ѡ��һ���ɡ���3������������ʱ���ɲ������ѱ������к���TiO(C2O4)22-����b���������Ű�ˮ�IJ��ϼ���C2O42-Ũ������������ƿ����ж�a��Ӧ�ѵ���ʽΪTiOC2O4�����ݳ����Ļ�ѧʽ����д�����ӷ���ʽTiO(C2O4)22-+ Ba2+ + 4H2O = BaTiO(C2O4)2��4H2O������4���ݹ���֪ϴ��Һ�п��ܺ���Cl����������AgNO3��Һ���鼴�ɡ���5������������̿��Cl2�жϲ���ΪTiCl4��CO�����Ի�ѧ����ʽΪTiO2+2C+2Cl2![]() TiCl4+2CO������BaSO4������Һ��c(Ba2+)��c(SO42-)��Ϊxmol��L-1����KSP(BaSO4)=c(Ba2+)c(SO42-)֪��x2=1.0��10-10(mol��L-1)2�������c(Ba2+)=1.0��10-5mol��L-1������Q(BaCO3)=c(Ba2+)c(CO32-)=1.0��10-5mol��L-1��c(CO32-)>KSP(BaCO3)����c(CO32-)>2.58��10-9(mol��L-1)2��1.0��10-5mol��L-1=2.58��10-4mol��L-1��
TiCl4+2CO������BaSO4������Һ��c(Ba2+)��c(SO42-)��Ϊxmol��L-1����KSP(BaSO4)=c(Ba2+)c(SO42-)֪��x2=1.0��10-10(mol��L-1)2�������c(Ba2+)=1.0��10-5mol��L-1������Q(BaCO3)=c(Ba2+)c(CO32-)=1.0��10-5mol��L-1��c(CO32-)>KSP(BaCO3)����c(CO32-)>2.58��10-9(mol��L-1)2��1.0��10-5mol��L-1=2.58��10-4mol��L-1��