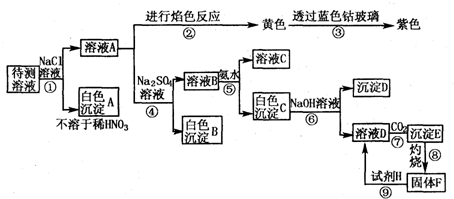
��Ŀ����
ij����Һ�п��ܺ���(1)ʵ���в�������������������������(�����ʽ)��?
(2)ԭ��Һ��һ�����е�������������������������������������
���ܺ��е���������������������֤�����Ӵ��ڵķ����ǣ�������������������������������
(3)ԭ��Һ��һ�������е�������������������������?
(4)�ڳ����м�������NaOH��Һ�����������ܽ⣬������Ӧ�����ӷ���ʽΪ��_________________��
(1)NH3(2)![]() ��M2+?��Al3+?��Na+ͨ����ɫ��Ӧʵ�飬����Na+���ڣ���ɫ��Ӧ�ʻ�ɫ?
��M2+?��Al3+?��Na+ͨ����ɫ��Ӧʵ�飬����Na+���ڣ���ɫ��Ӧ�ʻ�ɫ?
(3)Fe2+��Cu2+��![]() ?
?
(4)Al(OH)3 + OH-��![]() + 2H2O?
+ 2H2O?
����������Һ�м���NaOH������,�а�ɫ��������ܺ���Mg2+��Al3+���϶���Fe2+?��Cu2+���д̼�����ζ������������NH+4��������м������NaOH�����������ܽ⣬�϶���Mg2+��Al3+����![]() ��
��

��ϰ��ϵ�д�
�����Ŀ
ij����Һ�����ܺ������������еļ��֣�Fe3+��NH4+��Mg2+��Ba2+��NO3-��HCO3-��SO42-��S2O32-��������Һ�ֳ����ȷݣ���������ʵ�飺
���ڵ�һ����Һ�м�������ϡ���ᣬ�õ���ɫ��Һͬʱ������ɫ�����3.2g����ɫ������
���ڵڶ�����Һ�м�������������������Һ���õ���ɫ��������Һ��ּ��Ⱥ�����������ʹʪ��ĺ�ɫʯ����ֽ������
���ڵ�������Һ�м���������ϡ������Ȼ�����Һ�����յõ�69.9g��ɫ������
��֪��8NO3-+2H++3S2O32-=6SO42-+8NO��+H2O
����˵������ȷ���ǣ�������
���ڵ�һ����Һ�м�������ϡ���ᣬ�õ���ɫ��Һͬʱ������ɫ�����3.2g����ɫ������
���ڵڶ�����Һ�м�������������������Һ���õ���ɫ��������Һ��ּ��Ⱥ�����������ʹʪ��ĺ�ɫʯ����ֽ������
���ڵ�������Һ�м���������ϡ������Ȼ�����Һ�����յõ�69.9g��ɫ������
��֪��8NO3-+2H++3S2O32-=6SO42-+8NO��+H2O
����˵������ȷ���ǣ�������
| A������ʵ��ٿɵó��Ľ����ǣ�ԭ��Һ�к�S2O32-����Fe3+��NO3- | B��ԭ��Һ�п϶����ڵ������ǣ�S2O32-��NH4+��Mg2+��SO42- | C������ȷ���Ƿ���ڵ������ǣ�HCO3-��SO42- | D��ʵ��۵õ���ɫ����ΪBaSO4 |