��Ŀ����
100mLij��ɫ����Һ�п��ܺ���Fe3+��Ag+��Al3+��Ba2+��Mg2+��K+��Na+�����ӣ���������ʵ�飨�����Լ�����������
��1����������ʵ�飬�ܷ��жϴ�����Һ���Ƿ���Na+ ����д���ܡ����ܡ����������� ��
��2����Һ��һ�������������ӣ������п��ܴ��ڵ�һ���������� ��
��3��д�����г�������ɣ��û�ѧʽ��ʾ����
��4����Ӧ�����ӷ���ʽΪ ��
��5����ʵ���н�����ɫ��Ӧ�����˵Ȳ���ʱ��ԭ��Һ����ĺ��Բ��ƣ�ʵ���ֻ��ó���C����F�������ֱ�Ϊng��mg���ݴ���ȷ��ԭ��Һ����Щ���ӵ����ʵ���Ũ�ȣ�д�������ʽ ��

��1����������ʵ�飬�ܷ��жϴ�����Һ���Ƿ���Na+
��2����Һ��һ�������������ӣ������п��ܴ��ڵ�һ����������
��3��д�����г�������ɣ��û�ѧʽ��ʾ����
| A | B | C | E |
��5����ʵ���н�����ɫ��Ӧ�����˵Ȳ���ʱ��ԭ��Һ����ĺ��Բ��ƣ�ʵ���ֻ��ó���C����F�������ֱ�Ϊng��mg���ݴ���ȷ��ԭ��Һ����Щ���ӵ����ʵ���Ũ�ȣ�д�������ʽ
��������1�������ӵļ���Ҫͨ����ɫ��Ӧ��ʾ��ɫ�����飻
��2�����ӵļ��鷽���Լ������������ȷ�����ڵ����ӺͲ����ڵ����ӣ�
��3���������ӵ��������ӷ�Ӧ�Լ����������������ȷ����������������
��4���������������ܽ������������У��õ�ƫ��������Һ��
��5�����ݳ������������Ԫ���غ�������ӵ����ʵ��������������ʵ���Ũ�ȣ�
��2�����ӵļ��鷽���Լ������������ȷ�����ڵ����ӺͲ����ڵ����ӣ�
��3���������ӵ��������ӷ�Ӧ�Լ����������������ȷ����������������
��4���������������ܽ������������У��õ�ƫ��������Һ��
��5�����ݳ������������Ԫ���غ�������ӵ����ʵ��������������ʵ���Ũ�ȣ�
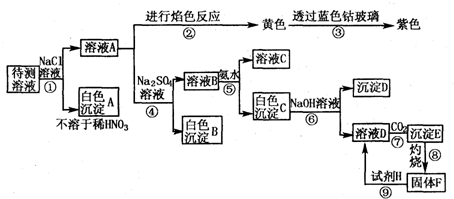
����⣺��1������Һ�м����Ȼ��ƣ�������ɫ����������ij�����һ�����������ӣ�����A���Ȼ�����������ҺA ������ɫ��Ӧʵ�飬��ʾ��ɫ�����������ӣ�����ǰһ����������Ȼ����к��������ӣ����Բ���ȷ������Һ���Ƿ��������ӣ�
�ʴ�Ϊ�����ܣ���ҺA��ɫ��Ӧ�ʻ�ɫ��������Ϊ����Һ�м�����NaCl��Ե�ʣ�
��2������ɫ����Һ��һ�����Ậ��Fe3+����A�м��������ƣ�������ɫ����B����BΪ���ᱵ��һ�����б����ӣ���B��Һ�м��백ˮ�������İ�ɫ����D������ҺD�мӶ�����̼�������İ�ɫ�������������������Գ���E������������������ɫ����Dֻ����������þ��C��������þ�����������Ļ������е��������������ܽ������������У��õ�D��Һ��ƫ��������Һ������һ������þ���Ӻ������ӣ�����һ�����е������ǣ�Ag+��Al3+��Ba2+��Mg2+���ܺ������ӹ����������ֻ����������ӣ�����������Ӻ�����������Ҳ���Թ��棬�ʴ�Ϊ��NO3-����������ӣ���
��3�����ݣ�1����2���ķ���������A���Ȼ�������������BΪ���ᱵ������E����������������C��������þ�����������Ļ����ʴ�Ϊ��
��
��4���������������ܽ������������У��õ�ƫ��������Һ����OH-+Al��OH��3=AlO2-+2H2O���ʴ�Ϊ��OH-+Al��OH��3=AlO2-+2H2O��
��5��ʵ���ֻ��ó���C��Mg��OH��2��Al��OH��3����F�������������ֱ�Ϊng��mg��������Ԫ���غ㣬mg��������AlԪ�ص����ʵ�����
��2mol�����������ӵ����ʵ�������ȵģ����������ӵ�Ũ��c��Al3+��=
mol/L��������Ԫ���غ㣬����������������
��2mol��78g������Mg��OH��2��Al��OH��3
����������ng������������þ�����ǣ�n-
��2mol��78��g������þ���ӵ����ʵ�����
mol��Ũ����c��Mg2+��=
mol/L���ʴ�Ϊ��c��Al3+��=
mol/L��c��Mg2+��=
mol/L��
�ʴ�Ϊ�����ܣ���ҺA��ɫ��Ӧ�ʻ�ɫ��������Ϊ����Һ�м�����NaCl��Ե�ʣ�
��2������ɫ����Һ��һ�����Ậ��Fe3+����A�м��������ƣ�������ɫ����B����BΪ���ᱵ��һ�����б����ӣ���B��Һ�м��백ˮ�������İ�ɫ����D������ҺD�мӶ�����̼�������İ�ɫ�������������������Գ���E������������������ɫ����Dֻ����������þ��C��������þ�����������Ļ������е��������������ܽ������������У��õ�D��Һ��ƫ��������Һ������һ������þ���Ӻ������ӣ�����һ�����е������ǣ�Ag+��Al3+��Ba2+��Mg2+���ܺ������ӹ����������ֻ����������ӣ�����������Ӻ�����������Ҳ���Թ��棬�ʴ�Ϊ��NO3-����������ӣ���
��3�����ݣ�1����2���ķ���������A���Ȼ�������������BΪ���ᱵ������E����������������C��������þ�����������Ļ����ʴ�Ϊ��
| A | B | C | D |
| AgCl | BaSO4 | Mg��OH��2��Al��OH��3 | Al��OH��3 |
��4���������������ܽ������������У��õ�ƫ��������Һ����OH-+Al��OH��3=AlO2-+2H2O���ʴ�Ϊ��OH-+Al��OH��3=AlO2-+2H2O��
��5��ʵ���ֻ��ó���C��Mg��OH��2��Al��OH��3����F�������������ֱ�Ϊng��mg��������Ԫ���غ㣬mg��������AlԪ�ص����ʵ�����
| m |
| 102 |
2��
| ||
| 0.1 |
| m |
| 102 |
����������ng������������þ�����ǣ�n-
| m |
| 102 |
(n-
| ||
| 58 |
n-2��
| ||
| 58��0.1 |
2��
| ||
| 0.1 |
n-2��
| ||
| 58��0.1 |
������������һ�����Ӽ������֪ʶ���ۺϿ����⣬��������������������Ѷȣ��ۺ���ǿ���Ѷȴ�

��ϰ��ϵ�д�
�����Ŀ