��Ŀ����
������ͼ����ʯī���缫�ĵ����У��׳���Ϊ500mL��ijһ���ʵ���ɫ��Һ���ҳ���Ϊ500mLϡ���ᣬ�պ�K1���Ͽ�K2���е�⣬�۲쵽A�缫�����к�ɫ�Ĺ�̬�������ɣ�B�缫����ɫ�������ɣ�����Һ�е�ԭ��������ȫ����ֹͣ��⣬ȡ��A�缫��ϴ�ӡ�����������缫����6.4g��
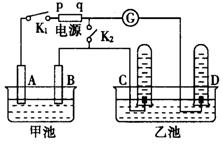
��ش��������⣺
��1��B�缫������Ӧ�ĵ缫��Ӧʽ ��
C�缫������Ӧ�ĵ缫��Ӧʽ .
��2���׳ص��ʱ��Ӧ�����ӷ���ʽ ��
��3���׳ص�����Һ��c(H+)Ϊ ��Ҫʹ������Һ�ָ������ǰ��״̬��������� ��������Ϊ g����������ǰ����Һ��������䣩
��4���������ʵ��ȷ���׳�ԭ��Һ�п��ܺ��е�������ӣ�Ҫ��������ֿ��ܵļ��裬�ֱ�д����֤�����ּ���IJ������衢ʵ�������ʵ�����
�ټ���һ�� ��
![]() �ڼ������ ��
�ڼ������ ��
��5�����ٽ�K1�Ͽ����պ�K2������������ ��ָ���Ƿ�ᷢ��ƫת��������ʲô��
![]()
![]()
![]() ��1��4OH��-4e�� 2H2O+O2�� 2H++2e�� H2��
��1��4OH��-4e�� 2H2O+O2�� 2H++2e�� H2��
��2��2Cu2++2H2O 2Cu+O2��+4H+
��3��0.4mol/L CuO 8
��4���ټ���ԭ��Һ�е��������ΪSO42����ȡ�������Һ�������м���BaCl2��Һ�����а�ɫ������������ԭ��Һ�к�SO42�� �ڼ���ԭ��Һ�е��������ΪNO3����ȡ�������Һ�������м���Cu�ȣ���Cu�ܽ⣬������ɫ�������ɣ��ڿ����б�Ϊ����ɫ������NO3��
![]() ��5��ƫת ��K1���ر�K2�� ��ָ���ƫת�������γ�H2��O2ȼ�ϵ�ء�
��5��ƫת ��K1���ر�K2�� ��ָ���ƫת�������γ�H2��O2ȼ�ϵ�ء�

 ����ͼ��ʯī���缫�ĵ����У�����500mL��һ�����ʵ�ij��ɫ��������Һ���е�⣬�۲쵽A�缫�����к�ɫ�Ĺ�̬�������ɣ�B�缫����ɫ�������ɣ���ش��������⣺
����ͼ��ʯī���缫�ĵ����У�����500mL��һ�����ʵ�ij��ɫ��������Һ���е�⣬�۲쵽A�缫�����к�ɫ�Ĺ�̬�������ɣ�B�缫����ɫ�������ɣ���ش��������⣺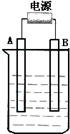 ����ͼ��ʯī���缫�ĵ����У�����500mL��һ�����ʵ�ij��ɫ��Һ���е�⣬�۲쵽A�缫�����к�ɫ�Ĺ�̬�������ɣ�B�缫����ɫ�������ɣ���ش��������⣺
����ͼ��ʯī���缫�ĵ����У�����500mL��һ�����ʵ�ij��ɫ��Һ���е�⣬�۲쵽A�缫�����к�ɫ�Ĺ�̬�������ɣ�B�缫����ɫ�������ɣ���ش��������⣺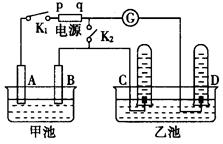
 �ڼ������ ��
�ڼ������ ��