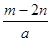��Ŀ����
��NA��ʾ����ӵ�������ֵ������˵������ȷ����
| A��һ�������£�2.3g��Na��ȫ��O2��Ӧ����3.6g����ʱʧȥ�ĵ�����Ϊ0.1NA |
| B��һ���������ú�1mol FeCl3����Һ�Ʊ�Fe(OH)3���壬����NA��Fe(OH)3���� |
| C����״���£�22.4L��CCl4�к��е�CCl4������ΪNA |
| D�����³�ѹ�£�2.24LCO��CO2��������к��е�̼ԭ����ĿΪ0.1NA |
A
���������
A��һ�������£�2.3g��Na��ȫ��O2��Ӧ����3.6g����ʱʧȥ�ĵ�����Ϊ0.1NA����ȷ��
B��һ���������ú�1mol FeCl3����Һ�Ʊ�Fe(OH)3���壬 �������Ӳ����ǵ������ڵģ����Բ��������ò���NA��Fe(OH)3���� ��
C����״���£�CCl4��Һ̬�ģ�����22.4L��CCl4�к��е�CCl4������Ϊ����NA��
D�����³�ѹ�£�2.24LCO��CO2������壬�����ʵ�������1mol���������к��е�̼ԭ����Ŀ��Ϊ0.1NA����ѡA��
����������٤�������dz������������ͣ���Ϥ�����ʵ����йص������������ʵ���Ũ�ȣ�����Ħ�����������������٤�������ȣ���������㲻�ѽ����
��𰢷��ӵ�����(NA)���������ʱ������ע������һЩϸ��֪ʶ�㣺
�ٱ�״���·��������ʣ�ˮ���塢SO3��CCl4���������顢CHCl3�Ȳ�����Vm=22��4L/mol�����ת��Ϊ���ʵ�����
�ڷ�����ԭ�Ӹ������⣺��������������������˫ԭ�ӵķ��ӣ�ϡ������(��ԭ�ӷ���)������(P4)������(O3)��
�۽ϸ��ӵ�������ԭ��Ӧ��ת�Ƶĵ�������Na2O2��H2O��Cl2��NaOH��KClO3�����ᡢͭ�����AgNO3�ȡ�

��ϰ��ϵ�д�
�����Ŀ
 �����ʵ���Ũ��֮��Ϊ ( )
�����ʵ���Ũ��֮��Ϊ ( )