��Ŀ����
| |||||||||||||||||||||||||||
�𰸣�
������
������
(1) |
4,VIIA |
(2) |
�ữ�ɷ�ֹCl2��Br2��ˮ��Ӧ |
(3) |
Br2��SO2��2H2O��4H+��2Br����SO42�� |
(4) |
�¶ȹ��ߣ�����ˮ������ˮ�ų�����������ˮ���ӣ��¶ȹ��ͣ��岻����ȫ�����������ʵ͡� |
(5) |
��Һ©��,�¿� |
(6) |
�Ȼ���ĺ�ˮ��Ȼ�����嵥�ʣ���Ũ�ȵͣ����ֱ������ԭ�ϣ���Ʒ�ɱ��ߡ�������������SO2���ա��Ȼ����Ĺ���ʵ������һ��Br2��Ũ�����̡� |

��ϰ��ϵ�д�
�����Ŀ
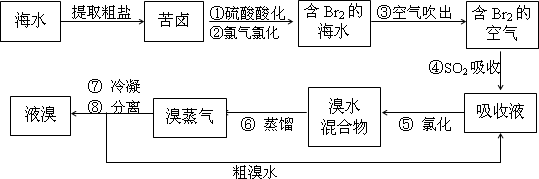
�������������գ���Ŀǰ����ˮ���塱������Ҫ����֮һ���乤���������£����в���ȷ���ǣ�������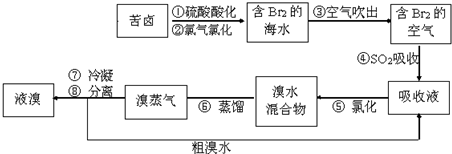

| A����������������ữ�ɷ�ֹCl2��Br2��ˮ��Ӧ | B������ܵ����ӷ���ʽBr2+SO2+H2O=2H++2Br-+SO42- | C���������ڱ���λ��4����VIIA�� | D���������������������õ�Һ������ˮ�Ļ������÷�Һ©�� |