��Ŀ����
��2011?�Ͼ�ģ�⣩�������������գ���Ŀǰ����ˮ���塱������Ҫ����֮һ���乤���������£�

��1���������ڱ���λ��
��2������ܵ����ӷ���ʽ��
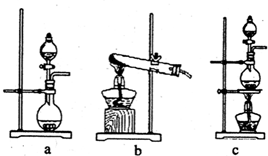
��3����֪��ķе���58.5�棬�������������У�������¶�Ϊ��Ҫ������80-90�森�¶ȹ�����Ͷ������������������ԭ��
��4���������������������õ�Һ������ˮ�Ļ������������ǵ�����ܶ����ܴ���ص���з��룮����ʵ���ҷ������������ķ���������������
��5��Ϊʲô��ֱ���ú���ĺ�ˮ��������õ�Һ�壬��Ҫ����������������SO2���ա��Ȼ�����

��1���������ڱ���λ��
����
����
���ڣ���A
��A
�壮��2������ܵ����ӷ���ʽ��
Br2+SO2+2H2O=4H++2Br-+SO42-
Br2+SO2+2H2O=4H++2Br-+SO42-
����3����֪��ķе���58.5�棬�������������У�������¶�Ϊ��Ҫ������80-90�森�¶ȹ�����Ͷ������������������ԭ��
�¶ȹ��ߣ�����ˮ�����ų���������ˮ���ӣ��¶ȹ��ͣ��岻����ȫ�����������ʵ�
�¶ȹ��ߣ�����ˮ�����ų���������ˮ���ӣ��¶ȹ��ͣ��岻����ȫ�����������ʵ�
����4���������������������õ�Һ������ˮ�Ļ������������ǵ�����ܶ����ܴ���ص���з��룮����ʵ���ҷ������������ķ���������������
��Һ©��
��Һ©��
������ʱҺ��ӷ��������¿�
�¿�
����Ͽڡ����¿ڡ����ų�����5��Ϊʲô��ֱ���ú���ĺ�ˮ��������õ�Һ�壬��Ҫ����������������SO2���ա��Ȼ�����
�Ȼ���ĺ�ˮ��Ȼ�����嵥�ʣ���Ũ�ȵͣ����ֱ������ԭ�ϣ���Ʒ�ɱ��ߡ�����������SO2���ա��Ȼ����Ĺ���ʵ������һ��Br2�ĸ�������
�Ȼ���ĺ�ˮ��Ȼ�����嵥�ʣ���Ũ�ȵͣ����ֱ������ԭ�ϣ���Ʒ�ɱ��ߡ�����������SO2���ա��Ȼ����Ĺ���ʵ������һ��Br2�ĸ�������
�����������������������ڹ�ҵ��ģ��ˮ����ij��÷���������һ�ֹ�������Ԥ�Ⱦ����ữ��Ũ����ˮ�У��������û�������ʹ֮��Ϊ�����壬�̶�ͨ�������ˮ���������崵����������ʹ�����������ռ���������������ת�����������Դﵽ������Ŀ�ģ�Ҳ���ǵõ������壮Ȼ�������������������õ���Ʒ�壬
��1���������ԭ������Ϊ35�����ԭ�Ӻ�������Ų��ж������ڱ��е�λ�ã�
��2��Br2��SO2����������ԭ��Ӧ����HBr��H2SO4���Դ���д���ӷ���ʽ��
��3��������80-90�棬��ֹ������Br2��ˮ�����ӷ���
��4�����÷�Һ�ķ�������Һ������ˮ�Ļ���Һ���ܶȴ���ˮ��
��5���Ȼ���ĺ�ˮ��Ȼ�����嵥�ʣ���Ũ�ȵͣ������ͣ�
��1���������ԭ������Ϊ35�����ԭ�Ӻ�������Ų��ж������ڱ��е�λ�ã�
��2��Br2��SO2����������ԭ��Ӧ����HBr��H2SO4���Դ���д���ӷ���ʽ��
��3��������80-90�棬��ֹ������Br2��ˮ�����ӷ���
��4�����÷�Һ�ķ�������Һ������ˮ�Ļ���Һ���ܶȴ���ˮ��
��5���Ȼ���ĺ�ˮ��Ȼ�����嵥�ʣ���Ũ�ȵͣ������ͣ�
����⣺��1�����ԭ������Ϊ35��ԭ�Ӻ�����4�����Ӳ㣬����������Ϊ7����λ�����ڱ��������ڢ�A�壬
�ʴ�Ϊ�����ģ���A��
��2��Br2��SO2����������ԭ��Ӧ����HBr��H2SO4����Ӧ�����ӷ���ʽΪBr2+SO2+2H2O=4H++2Br-+SO42-��
�ʴ�Ϊ��Br2+SO2+2H2O=4H++2Br-+SO42-��
��3���������������У�������¶�Ϊ��Ҫ������80-90�棬ӦΪ�¶ȹ��ߣ�����ˮ�����ų���������ˮ���ӣ� �¶ȹ��ͣ��岻����ȫ�����������ʵͣ��¶ȹ�����Ͷ�������������
�ʴ�Ϊ���¶ȹ��ߣ�����ˮ�����ų���������ˮ���ӣ� �¶ȹ��ͣ��岻����ȫ�����������ʵͣ�
��4��Һ������ˮ�Ļ����ֲ㣬���÷�Һ�ķ�������Һ������ˮ�Ļ���Һ���ܶȴ���ˮ���ӷ�Һ©���¿�������
�ʴ�Ϊ����Һ©�����¿ڣ�
��5���Ȼ���ĺ�ˮ��Ȼ�����嵥�ʣ���Ũ�ȵͣ������ͣ�Ӧ����������������SO2���ա��Ȼ����Ĺ��̽��и�����
�ʴ�Ϊ���Ȼ���ĺ�ˮ��Ȼ�����嵥�ʣ���Ũ�ȵͣ����ֱ������ԭ�ϣ���Ʒ�ɱ��ߡ�����������SO2���ա��Ȼ����Ĺ���ʵ������һ��Br2�ĸ������̣�
�ʴ�Ϊ�����ģ���A��
��2��Br2��SO2����������ԭ��Ӧ����HBr��H2SO4����Ӧ�����ӷ���ʽΪBr2+SO2+2H2O=4H++2Br-+SO42-��
�ʴ�Ϊ��Br2+SO2+2H2O=4H++2Br-+SO42-��
��3���������������У�������¶�Ϊ��Ҫ������80-90�棬ӦΪ�¶ȹ��ߣ�����ˮ�����ų���������ˮ���ӣ� �¶ȹ��ͣ��岻����ȫ�����������ʵͣ��¶ȹ�����Ͷ�������������
�ʴ�Ϊ���¶ȹ��ߣ�����ˮ�����ų���������ˮ���ӣ� �¶ȹ��ͣ��岻����ȫ�����������ʵͣ�
��4��Һ������ˮ�Ļ����ֲ㣬���÷�Һ�ķ�������Һ������ˮ�Ļ���Һ���ܶȴ���ˮ���ӷ�Һ©���¿�������
�ʴ�Ϊ����Һ©�����¿ڣ�
��5���Ȼ���ĺ�ˮ��Ȼ�����嵥�ʣ���Ũ�ȵͣ������ͣ�Ӧ����������������SO2���ա��Ȼ����Ĺ��̽��и�����
�ʴ�Ϊ���Ȼ���ĺ�ˮ��Ȼ�����嵥�ʣ���Ũ�ȵͣ����ֱ������ԭ�ϣ���Ʒ�ɱ��ߡ�����������SO2���ա��Ȼ����Ĺ���ʵ������һ��Br2�ĸ������̣�
���������⿼�������ȡ����Ŀ�Ѷ��еȣ�ע��ʵ��Ļ�������������ʵ�����������ע�����

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 ��2011?�Ͼ�ģ�⣩�����£���10mL 0.1mol?L-1NaOH��Һ����μ���0.1mol?L-1������Һ�����õζ�������ͼ��ʾ������˵����ȷ���ǣ�������
��2011?�Ͼ�ģ�⣩�����£���10mL 0.1mol?L-1NaOH��Һ����μ���0.1mol?L-1������Һ�����õζ�������ͼ��ʾ������˵����ȷ���ǣ������� ��2011?�Ͼ�ģ�⣩������һ����Ҫ�Ļ���ԭ�ϣ�
��2011?�Ͼ�ģ�⣩������һ����Ҫ�Ļ���ԭ�ϣ�