��Ŀ����
11�����л���A������O2���ܱ������г��ȼ�գ�����ֻ��H2O��CO2������ͨ��ŨH2SO4����������5.4g����ͨ����ʯ����ȫ���գ���������8.8g�����A�ķ�����Ϊ62����1���л���A�к��е�Ԫ����C��H��O��
��2���л���A�ķ���ʽ��C2H6O2��
��3����0.2mol���л���Aǡ����4.6g�Ľ�������ȫ��Ӧ�ų�H2�����ƶϸ��л���Ľṹ��ʽCH3OCH2OH��
���� ����ͨ��ŨH2SO4����������5.4g��ӦΪH2O����������n��H2O��=$\frac{5.4g}{18g/mol}$=0.3mol����ͨ����ʯ����ȫ���գ���������8.8g��ӦΪCO2����������n��CO2��=$\frac{8.8g}{44g/mol}$=0.2mol����֪�л�����N��C����N��H��=2��6����Ϊ������ӦΪC2H6����A�ķ�����Ϊ62��Ӧ������OԪ�أ���N��O��=$\frac{62-2��12-6}{16}$=2�������ʽΪC2H6O2����0.2mol���л���Aǡ����4.6g�Ľ�������ȫ��Ӧ�ų�H2��˵���л��ﺬ���ǻ�����n��Na��=$\frac{4.6g}{23g/mol}$=0.2mol������1���ǻ����Դ˽����⣮
��� �⣺��1������ͨ��ŨH2SO4����������5.4g��ӦΪH2O����������n��H2O��=$\frac{5.4g}{18g/mol}$=0.3mol����ͨ����ʯ����ȫ���գ���������8.8g��ӦΪCO2����������n��CO2��=$\frac{8.8g}{44g/mol}$=0.2mol����֪�л�����N��C����N��H��=2��6����Ϊ������ӦΪC2H6����A�ķ�����Ϊ62��Ӧ������OԪ�أ�
�ʴ�Ϊ��C��H��O��
��2���л�����N��C����N��H��=2��6����N��O��=$\frac{62-2��12-6}{16}$=2�������ʽΪC2H6O2��
�ʴ�Ϊ��C2H6O2��
��3����0.2mol���л���Aǡ����4.6g�Ľ�������ȫ��Ӧ�ų�H2��˵���л��ﺬ���ǻ�����n��Na��=$\frac{4.6g}{23g/mol}$=0.2mol�����л��ﺬ��1���ǻ���ӦΪCH3OCH2OH��
�ʴ�Ϊ��CH3OCH2OH��
���� ���⿼������ȼ�շ�ȷ���л������ʽ���л���ṹ���жϡ������ŵ����ʵȣ���Ŀ�Ѷ��еȣ�ע������ԭ���غ��ж��л���ķ���ʽ������

 ����С��ʿ���������ϵ�д�
����С��ʿ���������ϵ�д�| A�� | ֲ���ƻ����۳����Ʈ�� | B�� | ������������ԭúΪԭ�� | ||
| C�� | ������ˮ�������ŷ� | D�� | �����ŷŵ�β�� |

| A�� | ������̼���⡢����������Ԫ����� | B�� | ���ܷ���������Ӧ | ||
| C�� | ���Dz����͵��л��� | D�� | �������Ǹ߷��ӻ����� |
| A�� | �߷��ӻ������Ǵ����� | |
| B�� | ʯ�͵��ѻ���ú�ĸ������ڻ�ѧ�仯��ú��Һ�����������仯 | |
| C�� | ��һ������ζ������ζ�Ŀ������� | |
| D�� | �ƿ��Ա�����ʮ������ |
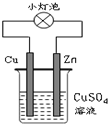
| A�� | ��װ��ʵ�ֻ�ѧ��ת��Ϊ���� | |
| B�� | ��װ����Cu2+��Cu�缫�ƶ� | |
| C�� | ��װ����Cu����Cu2+����������Ӧ����Cu | |
| D�� | ��װ����Zn���ϵĵ缫��ӦΪ��Zn-2e-�TZn2+ |
| A�� | ���ƺϽ�������ڿ����ӷ�Ӧ�ѵ��Ƚ����� | |
| B�� | MgO�����ʵ����²��� | |
| C�� | ���Ͻ��ܶ�С��ǿ�ȸߣ����Ƴ��������ֹǼܺͷɻ����� | |
| D�� | ���ƺ�������Ӧ�Ʊ��Ȼ��� |
| A�� | ����V2O5�����ı�÷�Ӧ���淴Ӧ���� | |
| B�� | ��2 mol SO2��2 mol O2�����ܱ������У���������2mol SO3 | |
| C�� | ��t1��t2ʱ�̣�SO2��g����Ũ�ȷֱ���C1��C2����ʱ����t1-t2�ڣ�SO2��g�����ĵ�ƽ������Ϊv=$\frac{{C}_{1}-{C}_{2}}{{t}_{2}-{t}_{1}}$ | |
| D�� | �÷�Ӧ�Ƿ��ȷ�Ӧ����SO2������һ������SO3������ |
| A�� | �� | B�� | ���Ը������ | C�� | ��ˮ | D�� | ����������ͭ |