��Ŀ����
����˵����ȷ����
- A.6.2g�����ƺ�7.8g�������ƻ�����������е�������ĿΪ0.7��6.02��1023
- B.1L 0.1mol?L-1Na2CO3��Һ�У�CO32-����ĿС��0.1��6.02��1023
- C.6.4 gSO2��һ��������������������ַ�Ӧ��ʧȥ�ĵ�����ĿΪ0.2��6.02��1023
- D.��״���£�22.4L CH4��CHCl3�Ļ���������еķ�����ĿΪ6.02��1023
B
���⿼�鰢��ӵ������������Ĺ�ϵ��6.2gNa2O�к������ӹ�0.3mol��7.8gNa2O2�к������ӹ�0.3mol�������������������Ϊ0.6��6.02��1023��A����Na2CO3Ϊǿ�������Σ��ᷢ��ˮ�⣬CO32-+H2O HCO3-+OH-��������Һ��CO32-����ĿС��0.1��6.02��1023��B��ȷ��6.4 gSO2��������Ӧ�ǿ��淴Ӧ��SO2�ڷ�Ӧ��ʧȥ�ĵ�����С��0.2��6.02��1023��C����״���£�CHCl3ΪҺ̬��D����
HCO3-+OH-��������Һ��CO32-����ĿС��0.1��6.02��1023��B��ȷ��6.4 gSO2��������Ӧ�ǿ��淴Ӧ��SO2�ڷ�Ӧ��ʧȥ�ĵ�����С��0.2��6.02��1023��C����״���£�CHCl3ΪҺ̬��D����
���⿼�鰢��ӵ������������Ĺ�ϵ��6.2gNa2O�к������ӹ�0.3mol��7.8gNa2O2�к������ӹ�0.3mol�������������������Ϊ0.6��6.02��1023��A����Na2CO3Ϊǿ�������Σ��ᷢ��ˮ�⣬CO32-+H2O
 HCO3-+OH-��������Һ��CO32-����ĿС��0.1��6.02��1023��B��ȷ��6.4 gSO2��������Ӧ�ǿ��淴Ӧ��SO2�ڷ�Ӧ��ʧȥ�ĵ�����С��0.2��6.02��1023��C����״���£�CHCl3ΪҺ̬��D����
HCO3-+OH-��������Һ��CO32-����ĿС��0.1��6.02��1023��B��ȷ��6.4 gSO2��������Ӧ�ǿ��淴Ӧ��SO2�ڷ�Ӧ��ʧȥ�ĵ�����С��0.2��6.02��1023��C����״���£�CHCl3ΪҺ̬��D���� 
��ϰ��ϵ�д�
�����Ŀ
Ӱ�컯ѧ��Ӧ���ʵ����غܶ࣬ij������ȤС����ʵ��ķ����о���Ӧ���ʵ��й����⣮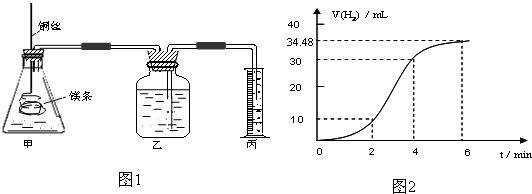
��1��ʵ��1 ̽��Mg�����ᷴӦ���ʵı仯���ɣ�ȡһ��þ������ɰֽ��ȥ���������Ĥ��ͭ˿����þ������װ�ü��У�ʹþ��������ƿ�ڵ����Ϊ2Lϡ���ᣨ�������У�þ�������ᷴӦ����H2������뷴Ӧʱ��Ĺ�ϵ������ͼ2��ʾ��
�ٴ�ͼ2�п���0-6min��ƽ����Ӧ��������ʱ����� ��������ţ�
A��0-2min B��2-4min C��4-6min
�������4-6min ʱ���ڣ���HCl��ʾ��ƽ����Ӧ����Ϊ ��������ͼ2����������ѻ���Ϊ��״���µ����������Һ����仯�ɺ��ԣ�
��ͼ1װ�ü�����þ��������ͭ˿��һ�����ϡ�����жԷ�Ӧ����Ӱ������˵����ȷ����
A���ӿ췴Ӧ���ʵ������������������� B��������Ӧ������������������
C����Ӱ�췴Ӧ���� D���ӿ췴Ӧ���ʵ�����������������С
��2��ʵ��2 ̽����Ũ�ȶ�MnO2��H2O2��Ӧ���ʵ�Ӱ��
��֪MnO2+H2O2+2H+�TMn2++O2��+2H2O����ȡ����MnO2���±��й����ʣ�����ͬ�¶��½���4��ʵ�飬�ֱ��¼�ռ�20.0mL��������ʱ�䣮
���ϱ���V1= mL��V3= mL��
����ͬѧ���ʵ��I������Ϊʵ����ĶԱ�ʵ�飬�������� ��
����ʵ����t2��t3��t4����ɵó���ʵ������� ��

��1��ʵ��1 ̽��Mg�����ᷴӦ���ʵı仯���ɣ�ȡһ��þ������ɰֽ��ȥ���������Ĥ��ͭ˿����þ������װ�ü��У�ʹþ��������ƿ�ڵ����Ϊ2Lϡ���ᣨ�������У�þ�������ᷴӦ����H2������뷴Ӧʱ��Ĺ�ϵ������ͼ2��ʾ��
�ٴ�ͼ2�п���0-6min��ƽ����Ӧ��������ʱ�����
A��0-2min B��2-4min C��4-6min
�������4-6min ʱ���ڣ���HCl��ʾ��ƽ����Ӧ����Ϊ
��ͼ1װ�ü�����þ��������ͭ˿��һ�����ϡ�����жԷ�Ӧ����Ӱ������˵����ȷ����
A���ӿ췴Ӧ���ʵ������������������� B��������Ӧ������������������
C����Ӱ�췴Ӧ���� D���ӿ췴Ӧ���ʵ�����������������С
��2��ʵ��2 ̽����Ũ�ȶ�MnO2��H2O2��Ӧ���ʵ�Ӱ��
��֪MnO2+H2O2+2H+�TMn2++O2��+2H2O����ȡ����MnO2���±��й����ʣ�����ͬ�¶��½���4��ʵ�飬�ֱ��¼�ռ�20.0mL��������ʱ�䣮
| ʵ���� | �� | �� | �� | �� |
| 10%H2O2�����/mL | 5.0 | 5.0 | V1 | V2 |
| 20%��������/mL | 0 | 0.5 | 1.0 | V3 |
| ˮ�����/mL | 15 | 14.5 | V4 | 13.5 |
| ����ʱ��t/s | t1 | t2 | t3 | t4 |
����ͬѧ���ʵ��I������Ϊʵ����ĶԱ�ʵ�飬��������
����ʵ����t2��t3��t4����ɵó���ʵ�������


 �ǽ���Ԫ�ص��ж����������NO��NO2��N2O4�ȣ�
�ǽ���Ԫ�ص��ж����������NO��NO2��N2O4�ȣ�