��Ŀ����
��9�֣�
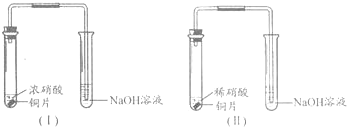
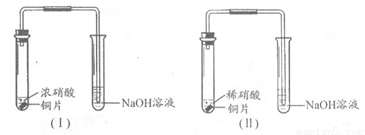
ij����ѧϰС��ֱ�����װ�â�̽��ͭ������ķ�Ӧ��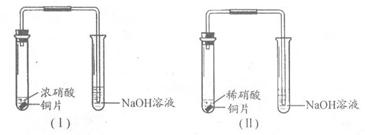
��1�����з�Ӧ�����ӷ���ʽΪ ��
���з�Ӧ�Ļ�ѧ����ʽΪ ��
�Ƚ�װ�â��еķ�Ӧ�����䲻ͬ���ǣ�
��
��2����С�����Ӧ�����Һ��С�����һ�����Ȼ����Ȼ��ȴ��ʵ���й۲쵽���Թ��ϲ�������ɫ�������������������Һ��ȫ�������������Թ��л��Һ�ʵ���ɫ���Թ�����ɫ������ʧ��
�١��Թ��ϲ�������ɫ����Ŀ���ԭ���� ��
�ڡ���������ԭ���� ��
�ۡ���Һ�ʵ���ɫ���Ŀ���ԭ���� ��
��1��Cu��4H����2NO3�� Cu2����2NO2����2H2O��2�֣�
3Cu��8HNO3 3Cu��NO3��2��2NO����4H2O��2�֣�
Cu��Ũ�����з�Ӧ���ң���������ɫ���壬��Һ��Ϊ��ɫ��Cu��ϡ�����з�Ӧ��������ɫ���壬������������ɫ����Һ��Ϊ��ɫ����2�֣�
��2�����������ȷֽ����NO2��2NO2 N2O4��ƽ�������ƶ���1�֣�
N2O4��ƽ�������ƶ���1�֣�
�ڵ��������ﱻNaOH��Һ���գ��Թ���ѹǿ���ͣ�1�֣�
��NO2��������NaOH��ȫ��Ӧ����Һ��ΪϡCu��NO3��2��Һ��1�֣�
����