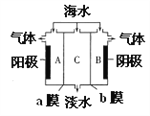
��Ŀ����
����Ŀ����O2��HClת��ΪCl2�������Ч�棬������Ⱦ����1����ͳ�ϸ�ת��ͨ������ͼ��ʾ�Ĵ���ѭ��ʵ�֣�

����
��Ӧ��Ϊ2HCl(g)+CuO(s)![]() H2O(g)+CuCl2(g) ��H1
H2O(g)+CuCl2(g) ��H1
��Ӧ������1 mol Cl2�ķ�Ӧ��Ϊ��H2�����ܷ�Ӧ���Ȼ�ѧ����ʽΪ_______________________(��Ӧ���á�H1�͡�H2��ʾ)��
��2������RuO2����������HClת��ΪCl2���ܷ�Ӧ���и��õĴ����ԣ�
��ʵ������һ��ѹǿ�£��ܷ�Ӧ��HClƽ��ת�������¶ȱ仯�Ĩ�HCl-T������ͼ��ʾ�����ܷ�Ӧ�ġ�H_____0 �������������������������A��B�����ƽ�ⳣ��K(A)��K(B)�нϴ����_______________��

��������ʵ������ѹ�����ʹѹǿ��������Ӧ��HCl��T���ߵ�ʾ��ͼ������Ҫ˵������__________��
��3�������д�ʩ����������ߨ�HCl����_____________��
A������n(HCl) B������n(O2) C��ʹ�ø��õĴ��� D����ȥH2O
��һ�������²�÷�Ӧ���̻���n(Cl2)���������£�
t/min | 0 | 2.0 | 4.0 | 6.0 | 8.0 |
n(Cl2)/10-3mol | 0 | 1.8 | 3.7 | 5.4 | 7.2 |
����2.0��6.0 min����HCl�����ʵ����仯��ʾ�ķ�Ӧ����Ϊ_______________����mol��min-1Ϊ��λ����
��4��Cl2��;�㷺��д��Cl2�Ʊ�Ư�۵Ļ�ѧ����ʽ__________________________________��
���𰸡� 4HCl��g��+O2��g��=2Cl2��g��+2H2O��g����H=2����H1+��H2�� �� K��A�� 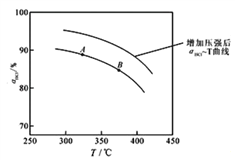 ;����ѹǿ��ƽ��������Ӧ�����ƶ�����HCl ������ͬ�¶��� HCl ��ƽ��ת���ʱ�֮ǰʵ��Ĵ� BD 1.8��10-3molmin-1 2Cl2+2Ca(OH)2=CaCl2+Ca(ClO)2+2H2O
;����ѹǿ��ƽ��������Ӧ�����ƶ�����HCl ������ͬ�¶��� HCl ��ƽ��ת���ʱ�֮ǰʵ��Ĵ� BD 1.8��10-3molmin-1 2Cl2+2Ca(OH)2=CaCl2+Ca(ClO)2+2H2O
��������(1)��ͼʾ��֪����������Ϊ��4HCl+O2=2Cl2+2H2O����Ӧ��Ϊ��2HCl(g)+CuO(s)![]() H2O(g)+CuCl2(s)��H1��
H2O(g)+CuCl2(s)��H1��
��Ӧ������1molCl2(g)�ķ�Ӧ��Ϊ��H2����Ӧ�Ȼ�ѧ����ʽΪ��CuCl2(g)+ ![]() O2(g)=CuO(s)+Cl2(g)��H2�����ݸ�˹����(��+��)��2�ɵ��ܷ�Ӧ���Ȼ�ѧ����ʽ��4HCl(g)+O2(g)=2Cl2(g)+2H2O(g)��H=2(��H1+��H2)���ʴ�Ϊ��4HCl(g)+O2(g)=2Cl2(g)+2H2O(g)��H=2(��H1+��H2)��
O2(g)=CuO(s)+Cl2(g)��H2�����ݸ�˹����(��+��)��2�ɵ��ܷ�Ӧ���Ȼ�ѧ����ʽ��4HCl(g)+O2(g)=2Cl2(g)+2H2O(g)��H=2(��H1+��H2)���ʴ�Ϊ��4HCl(g)+O2(g)=2Cl2(g)+2H2O(g)��H=2(��H1+��H2)��
(2)����ͼ��֪���¶�Խ�ߣ�ƽ��ʱHCl��ת����ԽС��˵�������¶�ƽ�����淴Ӧ�����ƶ���������ӦΪ���ȷ�Ӧ������H��0����ѧƽ�ⳣ����С����K(A)��K(B)���ʴ�Ϊ������K(A)��
������ӦΪ���������С�ķ�Ӧ������ѹǿ��ƽ��������Ӧ�����ƶ�����ͬ�¶���HCl��ƽ��ת���ʱ�֮ǰʵ��Ĵ�ѹ�����ʹѹǿ������Ӧ��HCl��T���ߵ�ʾ��ͼΪ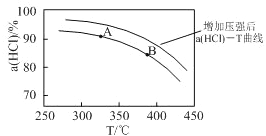 ���ʴ�Ϊ��
���ʴ�Ϊ�� ������ѹǿ��ƽ��������Ӧ�����ƶ�����HCl������ͬ�¶���HCl��ƽ��ת���ʱ�֮ǰʵ��Ĵ�
������ѹǿ��ƽ��������Ӧ�����ƶ�����HCl������ͬ�¶���HCl��ƽ��ת���ʱ�֮ǰʵ��Ĵ�
��A������n(HCl)��HClŨ������ƽ�����ƣ���HCl��ת���ʽ��ͣ���A����B������n(O2)������Ũ������ƽ�����ƣ�HCl��ת������ߣ���B��ȷ��C��ʹ�ø��õĴ������ӿ췴Ӧ���ʣ����̵���ƽ���ʱ�䣬��Ӱ��ƽ���ƶ���HCl��ת���ʲ��䣬��C����D����ȥ������H2O��������ƽ�����ƣ�HCl��ת��������D��ȷ����ѡ��BD��
(3)��2.0��6.0minʱ���ڣ�HClת�������ʵ���Ϊn����
2HCl(g)+1/2O2(g)�TH2O(g)+Cl2(g)
2������������������ 1
n������������ (5.4-1.8)��103mol
��á�n=7.2��10-3mol������v(HCl)=7.2��10-3mol/(6.0-2.0)min�T1.8��103mol/min���ʴ�Ϊ�� 1.8��10-3mol/min��
(4)�������������Ʒ�Ӧ�����Ȼ��ơ����������ˮ����Ӧ����ʽΪ2Cl2+2Ca(OH)2=CaCl2+Ca(ClO)2+2H2O���ʴ�Ϊ��2Cl2+2Ca(OH)2=CaCl2+Ca(ClO)2+2H2O��