��Ŀ����
����Ŀ��SCR��NSR��������Ч���Ͳ��ͷ������ڿ������������µ�NOx�ŷš�
��1��SCR��ѡ���Դ���ԭ������ԭ����

������[CO(NH2)2]ˮ��Һ�ȷֽ�ΪNH3��CO2����д��CO2�ĵ���ʽ___________________��
�ڷ�Ӧ����NH3��ԭNO2�����У�����ԭ���������������1mol��ת�Ƶ�������__________������NA��ʾ����
�۵�ȼ���к������ϸ�ʱ��β����SO2��O2�����»��γ�(NH4)2SO4��ʹ�����ж����û�ѧ����ʽ��ʾ(NH4)2SO4���γ�_______________________________________________��
��������ҺŨ��Ӱ��NO2��ת�����ⶨ��Һ�����أ�M=60 g��mol1�������ķ������£�ȡa g������Һ������������ȫת��ΪNH3������NH3�ù�����v1 mL c1 mol��L1 H2SO4��Һ������ȫ��ʣ��H2SO4��v2 mL c2 mol��L1 NaOH��Һǡ���кͣ���������Һ�����ʵ�����������________
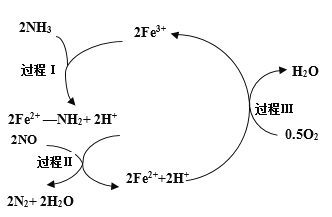
��2��NSR��NOx���滹ԭ������ԭ����NOx�Ĵ���ͻ�ԭ�ڲ�ͬʱ�ν�����У���ͼa��ʾ��

�� ͨ��BaO��Ba(NO3)2���ת��ʵ��NOx�Ĵ���ͻ�ԭ������NOx��������_________��
�� ��H2ģ��β���л�ԭ�������о���Ba(NO3)2�Ĵ���ԭ���̣��ù��̷��������У�ͼb��ʾ�ù����������Ũ����ʱ��ı仯��ϵ����һ����Ӧ���ĵ�H2��Ba(NO3)2�����ʵ���֮����__________��
�� ��ԭ�����У���ʱ�����Ц����N2O������ͬλ��ʾ�ٷ��о�����Ц���IJ�����NO�йء�������������15NO��NH3��һ��������Ӧʱ���õ���Ц����������15NNO�����÷�Ӧ�Ļ�ѧ����ʽ����������________________________ ______15NNO+ ________H2O
______15NNO+ ________H2O
���𰸡�![]() 24NA 2SO2��O2��4NH3��2H2O===2(NH4)2SO4
24NA 2SO2��O2��4NH3��2H2O===2(NH4)2SO4 ![]() % BaO 8��1 415NO��4NH3��3O2 4 6
% BaO 8��1 415NO��4NH3��3O2 4 6
��������
��1����CO2������ֻ���й��ۼ���ÿ��O��Cԭ�Ӽ��γ�2�Թ��õ��ӡ�
�ڷ�Ӧ����ʽΪ8NH3+6NO2=3N2(��ԭ����)+4N2(��������)+12H2O���ڴ˷�Ӧ�У���ת�Ƶ���8��3=24�����ɴ˿������ԭ���������������1molʱת�Ƶ���������
���γ�(NH4)2SO4ʱ����Ӧ��ΪSO2��O2��NH3��H2O��������Ϊ(NH4)2SO4��
��n(NH3)=2n(H2SO4)-n(NaOH)���������ݱ�����n(NH3)��������n(NH3)=2n[CO(NH2)2]�����������ص����ʵ���������������������
��2���� ��ͼ�пɿ���������NOx��������BaO��
�ڵ�һ����Ӧ���ĵ�H2��Ba(NO3)2��Ӧ�ķ���ʽΪ8H2+Ba(NO3)2=BaO+2NH3+5H2O���ɴ˿�ȷ��H2��Ba(NO3)2�����ʵ���֮�ȡ�
����ƽ�÷�Ӧ�Ļ�ѧ����ʽ��415NO+4NH3+3O2 415NNO+ 6H2O��
415NNO+ 6H2O��
��1����CO2������ֻ���й��ۼ���ÿ��O��Cԭ�Ӽ��γ�2�Թ��õ���, CO2�ĵ���ʽΪ![]() ����Ϊ
����Ϊ![]() ��
��
���ɷ�Ӧ����ʽ8NH3+6NO2=3N2(��ԭ����)+4N2(��������)+12H2O�ɿ�������ԭ���������������1mol����ת�Ƶ���8��3=24mol��ת�Ƶ�������24NA������Ϊ��24NA��
���γ�(NH4)2SO4ʱ����Ӧ����ʽΪ2SO2��O2��4NH3��2H2O==2(NH4)2SO4����Ϊ��2SO2��O2��4NH3��2H2O==2(NH4)2SO4��
��n(NH3)=2n(H2SO4)-n(NaOH)= (2c1V1 - c2V2 )��10-3mol��������n(NH3)= 2n[CO(NH2)2]��������n[CO(NH2)2]= ![]() ����������������Ϊ
����������������Ϊ
 ������
������![]() ��
��
��2���ٴ�ͼ�пɿ���������NOx��������BaO����Ϊ��BaO��
�ڵ�һ����Ӧ���ĵ�H2��Ba(NO3)2��Ӧ�ķ���ʽΪ8H2+Ba(NO3)2=BaO+2NH3+5H2O���ɴ˿�ȷ��H2��Ba(NO3)2�����ʵ���֮��8��1����Ϊ��8��1��
�۸��ݵ����غ㡢ԭ���غ���ƽ�÷�Ӧ�Ļ�ѧ����ʽΪ��4 415NNO+ 6H2O��
415NNO+ 6H2O��
����415NO��4NH3��3O2��4��6��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�