��Ŀ����
(20��)
18-I(6��)��֪�� �����Ҫ�ϳ�
���õ�ԭʼԭ�Ͽ�����
A. 2-��-l��3-����ϩ��2-��Ȳ B��1��3-���ϩ��2-��Ȳ
C��2��3-����-1��3-���ϩ����Ȳ D��2��3-����-l��3-����ϩ�ͱ�Ȳ
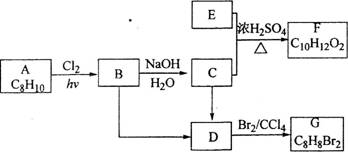
18-II(14��)A��G�����л���������ǵ�ת����ϵ���£�
��ش��������⣺
(1)��֪��6.0g������E��ȫȼ������8.8gC02��3.6gH20��E������������������ܶ�Ϊ30����E�ķ���ʽΪ_______________��
(2)AΪһȡ��������B�к���һ��������B����C�Ļ�ѧ����ʽΪ_______________��
(3)��B����D����C����D�ķ�Ӧ�����ֱ���_______________��_______________��
(4)��A����B����D����G�ķ�Ӧ���ͷֱ���_______________��_______________��
(5)F���������������У���ṹ��ʽΪ_______________��
(6)��G��ͬ���칹���У�������һ�����IJ���ֻ��һ�ֵĹ���___________�������к˴Ź�
������������壬�ҷ������Ϊl��1����_______________(��ṹ��ʽ)��
18-I A��D
18-II
(1)C2H4O2
(2)
(3)NaOH�Ĵ���Һ�����ȣ�Ũ���ᡢһ���¶���
(4)ȡ����Ӧ���ӳɷ�Ӧ
(5)
(6)7�֣�
����:

����������FeC2O4•2H2O�����������Լ�����Ӱ�������͵�ز�����������﮵���������֪��CO�����Ȼ��٣�PdCl2����Һ��Ӧ���ɺ�ɫ���ٷۡ��ش��������⣺
I����ȤС��Բ��������ķֽ�������ʵ���̽����
��1���������������ͨ��A������ʯ��ˮ��B���Ȼ��٣��۲쵽A�г���ʯ��ˮ������ǣ�B�г��ֺ�ɫ�������ɣ�����������˵������������� ��
��2��̽���ֽ�õ��Ĺ����������Ԫ�صĴ�����ʽ��
���������
����1��________�� ����2��FeO�� ����3��FeO��Fe�����
�����ʵ�鷽��֤������3��
��ѡ�Լ��� 1.0 mol•L��1���ᡢ3% H2O2��0.1 mol•L��1CuSO4��20% KSCN������ˮ��
|
ʵ�鲽�� |
��������� |
|
����1 �����Թ��м��������������ټ�������_________________������� |
����Һ��ɫ���Ըı䣬����_______���ɣ���֤���������ʴ��� |
|
����2�� ������1�еõ�����Һ���ˣ���������ˮϴ����ϴ��Һ��ɫ |
|
|
����3��ȡ����2�õ��������������Թ��У� �μ�___________________________________ _______________________________________
|
__________________________________ ___________________________________
|
II��ij����������Ʒ�к����������ᣨΪ�����ڼ��㣬���������в�����Ͳ�����Ӿ���C2O42�����棩�����õζ����ⶨ����Ʒ��FeC2O4�ĺ������ζ���Ӧ�ֱ��ǣ�5Fe2++MnO4��+8H+=5Fe3+ +Mn2++4H2O��5C2O42��+2MnO4��+16H+=10CO2��+2Mn2++8H2O��
��3��ʵ�鷽�����Ϊ��
�ٽ�ȷ������0.20g����������Ʒ����250 mL��ƿ�ڣ���������2 mol/L��H2SO4��Һ��ʹ��Ʒ�ܽ⣬������70�����ң�������Ũ��Ϊ0.02000 mol/L�ĸ�����ر���Һ�ζ����յ㣬��¼���ݡ��ظ��ζ�2�Ρ�ƽ������V1 mL��
���������ζ����Һ�м���������Zn�ۺ�����2 mol/L��H2SO4��Һ�����5��8min����KSCN��Һ�ڵ�ΰ��ϼ�����Һ��ֱ����Һ�����̱�졣����Һ��������һ����ƿ�У�������0.02000 mol/L�ĸ�����ر���Һ�ζ����յ㣬��¼���ݡ��ظ��ζ�2�Ρ�ƽ������V2 mL��
����ijС���һ�βⶨ���ݼ�¼���£� V1= 18.90mL��V2=6.20mL���������ݼ���0.20g ��Ʒ�У�n��Fe2+��= �� n��C2O42����= ��FeC2O4 ����������Ϊ ����ȷ��0.01%��FeC2O4��ʽ��Ϊ144��
��֪��N2��g����3H2��g��![]() 2NH3��g����H����92.4kJ/mol������Ŀǰ�ձ�ʹ�õ��˹��̵��ķ�������ش��������⣺
2NH3��g����H����92.4kJ/mol������Ŀǰ�ձ�ʹ�õ��˹��̵��ķ�������ش��������⣺
(1)450��ʱ����һ��2L���ܱ������г���2. 6mol H2��1mol N2�� ��Ӧ�����ж�NH3��Ũ�Ƚ��м�⣬�õ����������±���ʾ��
| ʱ��/min | 5 | 10 | 15 | 20 | 25 | 30 |
| c(NH3)/mol·L—1 | 0.08 | 0.14 | 0.18 | 0.20 | 0.20 | 0.20 |
�ٴ������¸÷�Ӧ�Ļ�ѧƽ�ⳣ��K=_______________����Ӧ�ﵽƽ�������ƽ����ϵ�м���H2��N2��NH3��2mol����ʱ�÷�Ӧ��v��N2����_______________v��N2��������д��>������=����<��=����
�����ı�ijһ����������ƽ��ʱn(H2)=1.60mol ������˵����ȷ����_____________��
A.ƽ��һ�������ƶ� B.�������������м�����һ������H2����
C.�����ǽ������������¶� D.��������С�����������
��2��450��ʱ������һ�ܱ������н��������ϳɰ��ķ�Ӧ�������ʵ���ʼŨ�Ⱥ�ƽ��Ũ�����±���ʾ��
| N2 | H2 | NH3 | |
| ��ʼŨ�ȣ�mol/L�� | 0.2 | 0.3 | 0.2 |
| ƽ��Ũ�ȣ�mol/L�� | a | b | c |
��ش�
��a��ȡֵ��Χ�ǣ�_______________��
��������ѧ����ʽ��ʾ������֮��Ĺ�ϵ��
(I)a��b�Ĺ�ϵ��_______________��(��)a��b��c�Ĺ�ϵ��_______________��
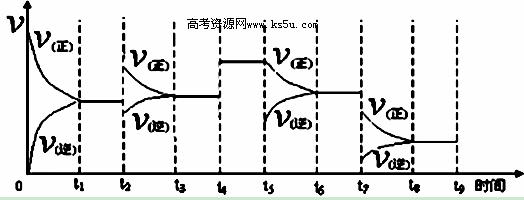
�۷�Ӧ�ﵽƽ��ı�ijһ�����������Ӧ������ʱ��Ĺ�ϵ����ͼ��ʾ������t2﹑t7ʱ������Ӧ��ʵ�������ı�ֱ��ǣ�t2 ��t7 ��
 �����Ҫ�ϳ�
�����Ҫ�ϳ� ���õ�ԭʼԭ�Ͽ�����
���õ�ԭʼԭ�Ͽ�����
 F���������������У���ṹ��ʽΪ_______________��
F���������������У���ṹ��ʽΪ_______________�� �����Ҫ�ϳ�
�����Ҫ�ϳ� ���õ�ԭʼԭ�Ͽ�����
���õ�ԭʼԭ�Ͽ�����