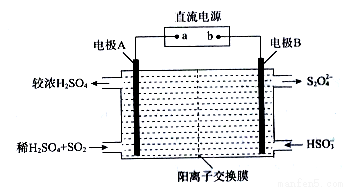
��Ŀ����
��NAΪ�����ӵ�������ֵ������˵����ȷ���ǣ� ��
A. ��1molFeCl3�ı�����Һ�����ˮ�еõ�������ĿΪNA
B. �ڱ�״���£�22.4LCl2��HCl�Ļ�������к��еķ�������Ϊ2��6.02��1023
C. ���³�ѹ�£�7.8gNa2S�����7.8gNa2O2�����к��е���������Ŀ��Ϊ0.1NA
D. ��״���£�Na2O2��������CO2��Ӧ����2.24LO2��ת�Ƶ�����Ϊ0.4NA
��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ


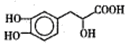

 b mol C.
b mol C.  a mol D. 2a mol
a mol D. 2a mol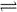 2NO(g) ��H��0
2NO(g) ��H��0