ЬтФПФкШн
ЁОЬтФПЁПФГбЇЯАаЁзщгћгУЛЏбЇЗНЗЈВтСПвЛИіВЛЙцдђШнЦїЕФЬхЛ§ЁЃАб23.4g NaClЗХШы200mLЩеБжаЃЌМгШы100mLеєСѓЫЎЃЌД§NaClЭъШЋШмНтКѓЃЌНЋШмвКШЋВПзЊвЦЕНИУШнЦїжаЃЌгУеєСѓЫЎЯЁЪЭжСЭъШЋГфТњИУШнЦїЃЌДгжаШЁГіШмвКl00mL ЃЌИУ100mLШмвКтљКУгы50 mL 0.1 mol/L AgNO3ЕФШмвКЭъШЋЗДгІЁЃ
ЃЈ1ЃЉЧѓ100mL NaClШмвКЕФЮяжЪЕФСПХЈЖШ_______________ЁЃ
ЃЈ2ЃЉЪдМЦЫуИУШнЦїЕФЬхЛ§_________________ЁЃ
ЁОД№АИЁП 0.05mol/L 8L
ЁОНтЮіЁПЪдЬтЗжЮіЃКЃЈ1ЃЉ NaCl+ AgNO3=AgClЁ§+NaNO3ЃЌИљОн![]() МЦЫу NaClШмвКЕФЮяжЪЕФСПХЈЖШЃЛЃЈ2ЃЉИљОн
МЦЫу NaClШмвКЕФЮяжЪЕФСПХЈЖШЃЛЃЈ2ЃЉИљОн![]() МЦЫуШнЦїЕФЬхЛ§ЁЃ
МЦЫуШнЦїЕФЬхЛ§ЁЃ
НтЮіЃКЃЈ1ЃЉ NaCl+ AgNO3=AgClЁ§+NaNO3ЃЌ ![]() ЃЌ
ЃЌ ![]()
![]() =0.05mol/LЃЛ
=0.05mol/LЃЛ
ЃЈ2ЃЉ23.4g NaClЕФЮяжЪЕФСПЪЧ![]() ЃЌЩшШнЦїЕФЬхЛ§ЪЧvLЃЌИљОн
ЃЌЩшШнЦїЕФЬхЛ§ЪЧvLЃЌИљОн![]() ЃЌv=
ЃЌv=![]() 8LЁЃ
8LЁЃ

ЁОЬтФПЁПЙигкБНМзЫсЕФжиНсОЇЪЕбщЃЌЦфНсТлЛђНтЪЭДэЮѓЕФЪЧ
бЁЯю | ЪЕбщВНжш | ЪЕбщЯжЯѓ | НсТлЛђНтЪЭ |
A | ГЃЮТШмНт | БНМзЫсМИКѕВЛШм | БНМзЫсГЃЮТЪБВЛШмгкЫЎЛђЮЂШмгкЫЎ |
B | МгШШШмНт | БНМзЫсЭъШЋШмНт | ЮТЖШЩ§ИпЃЌБНМзЫсШмНтЖШдіДѓ |
C | ГУШШЙ§ТЫ | Й§ТЫЪБАщгаОЇЬхЮіГі | ДЫОЇЬхЮЊдгжЪЫљаЮГЩ |
D | РфШДНсОЇЃЌТЫГіОЇЬх | еызДОЇЬх | еызДОЇЬхЮЊБНМзЫс |
A. A B. B C. C D. D
ЁОЬтФПЁП(1)бЁдёЪЪЕБЛЏбЇЪдМСГ§ШЅЯТСаЮяжЪЫљКЌдгжЪ(РЈКХФкЮЊдгжЪ)ЃЌЪЙФПБъЮяжЪЕУЕНЬсДПЃЛ
ЛьКЯЮя | ЪдМС | ЗжРыЗНЗЈ | |
Ђй | БН(БНЗг) | ______ | ______ |
Ђк | ввШВЃЈСђЛЏЧтЃЉ | ______ | ______ |
(2)ИјЯТСагаЛњЮяУќУћЃК
Ђй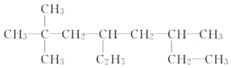 __________ЃЛ
__________ЃЛ
Ђк![]() _____________ЁЃ
_____________ЁЃ
