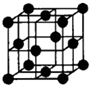
��Ŀ����
��֪A��B��C��D��EΪ���ֶ����ڲ�ͬ�����Ԫ�أ���ԭ���������ε�����A��E����ԭ������������֮����Bԭ��������������2����C��E����ԭ������������֮����Dԭ��������������2����A��E�γɵĻ���������е�������Ϊ18��Dԭ�Ӻ������������Ų�ʽΪnsnnpn+2����ش���������
��1��B��C��D����Ԫ�صĵ�һ�����ܽϴ����
��2��A������C������һ�������¿ɻ���Ϊ�ң������£���pH=a���ҵ�ˮ��Һ�м�������pH=b�ļ�ˮ��Һ����a+b=14��������ú���Һ��pH
��3��A��B��Ԫ��Ҳ���γɶ��ֻ����������Է���������С�ķ���Ϊ������D�ĵ��ʺͱ����Թ���ԭ��أ�����ö�Ķ��Ե缫����ŨKOH��Һ����X��ͨ�뻯�������Y��ͨ��D�ĵ��ʣ���X���ĵ缫��ӦʽΪ
��4����ҵ����EԪ�ص��ʵĻ�ѧ����ʽΪ
��5�����³�ѹ�£���A��B��D�����Һ̬����J������2.3gJ��������D�ĵ��ʳ�ַ�Ӧ�����ɱ�״����2.24L BD2�����2.7gA2DҺ�壬ͬʱ�ų�68.35kJ���������÷�Ӧ���Ȼ�ѧ����ʽΪ
��1��B��C��D����Ԫ�صĵ�һ�����ܽϴ����
N
N
����дԪ�ط��ţ���2��A������C������һ�������¿ɻ���Ϊ�ң������£���pH=a���ҵ�ˮ��Һ�м�������pH=b�ļ�ˮ��Һ����a+b=14��������ú���Һ��pH
��
��
7���������������=����������Һ�и�������Ũ���ɴ�С��˳��Ϊc��NH4+����C��Cl-����c��OH-����c��H+��
c��NH4+����C��Cl-����c��OH-����c��H+��
����3��A��B��Ԫ��Ҳ���γɶ��ֻ����������Է���������С�ķ���Ϊ������D�ĵ��ʺͱ����Թ���ԭ��أ�����ö�Ķ��Ե缫����ŨKOH��Һ����X��ͨ�뻯�������Y��ͨ��D�ĵ��ʣ���X���ĵ缫��ӦʽΪ
CH4-8e-+10OH-=CO32-+7H20
CH4-8e-+10OH-=CO32-+7H20
����4����ҵ����EԪ�ص��ʵĻ�ѧ����ʽΪ
2NaCl+2H20
2NaOH+Cl2��+H2��
| ||
2NaCl+2H20
2NaOH+Cl2��+H2��
��
| ||
��5�����³�ѹ�£���A��B��D�����Һ̬����J������2.3gJ��������D�ĵ��ʳ�ַ�Ӧ�����ɱ�״����2.24L BD2�����2.7gA2DҺ�壬ͬʱ�ų�68.35kJ���������÷�Ӧ���Ȼ�ѧ����ʽΪ
C2H5OH��l��+3O2��g��=2CO2��g��+3H2O��l����H=-1367KJ/mol
C2H5OH��l��+3O2��g��=2CO2��g��+3H2O��l����H=-1367KJ/mol
����������Dԭ�Ӻ������������Ų�ʽΪnsnnpn+2�����Ƴ�n=2����s�ܼ��������2�����ӣ�����Dԭ�Ӻ������������Ų�ʽΪ2s22p2+2��DΪOԪ�أ�C��E����ԭ������������֮����Dԭ��������������2������C��E����ԭ������������֮��Ϊ12������C��E���ڵ�5�͵�7���壬��Cԭ������С��OԪ�أ�����CΪNԪ�أ�EΪF��ClԪ�أ���A��E�γɵĻ���������е�������Ϊ18�����Ƴ�EΪClԪ�أ�AΪHԪ�أ���A��E����ԭ������������֮����Bԭ��������������2������֪B����������Ϊ��1+7����2�T4����BΪCԪ�أ�A��B��C��D��E�ֱ�Ϊ��H��C��N��O��ClԪ�أ�
��1�����ݵ����ܵı仯���ɿ��ǣ�
��2��A������C������һ�������¿ɻ���Ϊ�ң���ΪNH3����ΪHCl��pH=a���ҵ�ˮ��Һ��c��H+��Ϊ10-a��c��OH-��=10a-14��
pH=b�ļ�ˮ��Һ��c��H+��=10-b����a+b=14����-b=a-14������ˮ��c��OH-����������c��H+����ȣ����ߵ������ϼ�������õ�һˮ�ϰ����Ȼ�淋Ļ��Һ�����ݵ������ˮ���ж���Һ����Լ�����Ũ�ȴ�С��ϵ��
��3��C��H��ɵ���Է���������С��Ϊ���飬����Ϊ���飬����ȼ�ϵ���У�����������ȼ�ϣ�
��4����ҵ�����������õ�ⱥ��ʳ��ˮ����
��5������ȼ�ղ��������ȼ�ϵ����������ȼ�ϵ����ʽ�������жϳ�J���ʣ��ɴ�д���Ȼ�ѧ����ʽ��
��1�����ݵ����ܵı仯���ɿ��ǣ�
��2��A������C������һ�������¿ɻ���Ϊ�ң���ΪNH3����ΪHCl��pH=a���ҵ�ˮ��Һ��c��H+��Ϊ10-a��c��OH-��=10a-14��
pH=b�ļ�ˮ��Һ��c��H+��=10-b����a+b=14����-b=a-14������ˮ��c��OH-����������c��H+����ȣ����ߵ������ϼ�������õ�һˮ�ϰ����Ȼ�淋Ļ��Һ�����ݵ������ˮ���ж���Һ����Լ�����Ũ�ȴ�С��ϵ��
��3��C��H��ɵ���Է���������С��Ϊ���飬����Ϊ���飬����ȼ�ϵ���У�����������ȼ�ϣ�
��4����ҵ�����������õ�ⱥ��ʳ��ˮ����
��5������ȼ�ղ��������ȼ�ϵ����������ȼ�ϵ����ʽ�������жϳ�J���ʣ��ɴ�д���Ȼ�ѧ����ʽ��
����⣺�������Ϸ����ƶϳ�����Ԫ��A��B��C��D��E�ֱ�Ϊ��H��C��N��O��ClԪ�أ�
��1��ͬ���ڵ����ܴ���������������ƣ���NԪ��P���Ϊ�����״̬�����ȶ������Ե�һ�����ܷ�����O��Ҫ�ʴ�Ϊ��N��
��2��A������C������һ�������¿ɻ���Ϊ�ң���ΪNH3����ΪHCl��pH=a���ҵ�ˮ��Һ��c��H+��Ϊ10-a��c��OH-��=
10a-14��
pH=b�ļ�ˮ��Һ��c��H+��=10-b����a+b=14����-b=a-14������ˮ��c��OH-����������c��H+����ȣ����ߵ������ϼ������
�õ�һˮ�ϰ����Ȼ�淋Ļ��Һ�����ݵ������ˮ���ж���Һ�Լ��ԣ�
����c��NH4+����C��Cl-����c��OH-����c��H+����
��������ʵ��������ģ�����c��Cl-����c��OH-����������Ũ�ȴ�С��ϵ��
c��NH4+����C��Cl-����c��OH-����c��H+����
�ʴ�Ϊ����c��NH4+����C��Cl-����c��OH-����c��H+����
��3������ȼ�ϵ���У�����ʧ���ӱ�ɶ�����̼������Խ��ʣ�������̼��Ӧ����CO32-��
������ӦʽΪ��CH4-8e-+10OH-=CO32-+7H20���ʴ�Ϊ��CH4-8e-+10OH-=CO32-+7H20��
��4����ҵ�����������õ�ⱥ��ʳ��ˮ������ѧ����ʽΪ��2NaCl+2H20
2NaOH+Cl2��+H2��
�ʴ�Ϊ��2NaCl+2H20
2NaOH+Cl2��+H2��
��5��2.7gΪH2O��������2.24L ΪCO2���壬
��n��H2O��=
=0.15mol��n��CO2��=
=0.1mol��
��n��H��=2n��H2O��=0.3mol����m��H��=0.3g��
m��C��=0.1mol��12g/mol=1.2g��
���������غ㶨�ɿɵã�
m�����J��=m��C��+m��O��+m��H����
��m��O��=2.3g-1.2g-0.3g=0.8g��
��ˣ�n��O��=
=0.05mol��
���ԣ��л���J�У�n��C����n��H����n��O��=0.1mol��0.3mol��0.05mol=2��6��1��
���л���J�ķ���ʽΪC2H6O��2.3gJ�����ʵ���Ϊ��
=0.05mol����1molJȼ�շų�����Ϊ��
68.35kJ��0.05mol=1367KJ/mol���ʴ�Ϊ��C2H5OH��l��+3O2��g��=2CO2��g��+3H2O��l����H=-1367KJ/mol��
��1��ͬ���ڵ����ܴ���������������ƣ���NԪ��P���Ϊ�����״̬�����ȶ������Ե�һ�����ܷ�����O��Ҫ�ʴ�Ϊ��N��
��2��A������C������һ�������¿ɻ���Ϊ�ң���ΪNH3����ΪHCl��pH=a���ҵ�ˮ��Һ��c��H+��Ϊ10-a��c��OH-��=
10a-14��
pH=b�ļ�ˮ��Һ��c��H+��=10-b����a+b=14����-b=a-14������ˮ��c��OH-����������c��H+����ȣ����ߵ������ϼ������
�õ�һˮ�ϰ����Ȼ�淋Ļ��Һ�����ݵ������ˮ���ж���Һ�Լ��ԣ�
����c��NH4+����C��Cl-����c��OH-����c��H+����
��������ʵ��������ģ�����c��Cl-����c��OH-����������Ũ�ȴ�С��ϵ��
c��NH4+����C��Cl-����c��OH-����c��H+����
�ʴ�Ϊ����c��NH4+����C��Cl-����c��OH-����c��H+����
��3������ȼ�ϵ���У�����ʧ���ӱ�ɶ�����̼������Խ��ʣ�������̼��Ӧ����CO32-��
������ӦʽΪ��CH4-8e-+10OH-=CO32-+7H20���ʴ�Ϊ��CH4-8e-+10OH-=CO32-+7H20��
��4����ҵ�����������õ�ⱥ��ʳ��ˮ������ѧ����ʽΪ��2NaCl+2H20
| ||
�ʴ�Ϊ��2NaCl+2H20
| ||
��5��2.7gΪH2O��������2.24L ΪCO2���壬
��n��H2O��=
| 2.7g |
| 18g/mol |
| 2.24L |
| 22.4L/mol |
��n��H��=2n��H2O��=0.3mol����m��H��=0.3g��
m��C��=0.1mol��12g/mol=1.2g��
���������غ㶨�ɿɵã�
m�����J��=m��C��+m��O��+m��H����
��m��O��=2.3g-1.2g-0.3g=0.8g��
��ˣ�n��O��=
| 0.8g |
| 16g/mol |
���ԣ��л���J�У�n��C����n��H����n��O��=0.1mol��0.3mol��0.05mol=2��6��1��
���л���J�ķ���ʽΪC2H6O��2.3gJ�����ʵ���Ϊ��
| 2.3g |
| 46g/mol |
68.35kJ��0.05mol=1367KJ/mol���ʴ�Ϊ��C2H5OH��l��+3O2��g��=2CO2��g��+3H2O��l����H=-1367KJ/mol��
�����������ۺϿ�����Ԫ���ƶϡ�Ԫ�������ɡ�����Ũ�ȴ�С�Ƚϡ���Ӧʽ����д�����ݣ��ѶȽϴؼ���Ԫ���ƶϣ�Dԭ�Ӻ������������Ų�ʽΪnsnnpn+2�ǽ���ͻ�ƿڣ����ڵ����ܵĴ�С��ϵ��Ҫע�������ԣ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
��֪A��B��C��D�ֱ���Cu��Ag��Fe��Al���ֽ����е�һ�֣���֪��A��C������ϡ���ᷴӦ�ų����壻��B��D�������η�Ӧ���û�������D����C��ǿ�Ӧ�ų����壬�ɴ˿����ƶ�A��B��C��D�����ǣ�������
| A��Fe��Cu��Al��Ag | B��Al��Cu��Fe��Ag | C��Cu��Ag��Al��Fe | D��Ag��Al��Cu��Fe |

 ��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ�
��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ� ����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�
����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�