��Ŀ����
��2013?����һģ��[��ѧһѡ��5�л���ѧ����]
���������ű������ڡ��ƹ������м�������ܻ����������ܻ��������������ܼ����� �ڹ�ҵ����;�dz��㷺������������������Σ�����������ܻ���������ܶ࣬����һ�֡��ܻ��������Ʊ�������ͼ��
��֪��
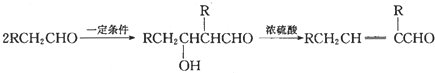
��ش��������}��
��1��д����ͼ��Ӧ�ķ�Ӧ���ͣ���
��2��д�����з���ʽ����

��3��д��D�����������Һ�����������ͬ���칹����
���������ű������ڡ��ƹ������м�������ܻ����������ܻ��������������ܼ����� �ڹ�ҵ����;�dz��㷺������������������Σ�����������ܻ���������ܶ࣬����һ�֡��ܻ��������Ʊ�������ͼ��
��֪��

��ش��������}��

��1��д����ͼ��Ӧ�ķ�Ӧ���ͣ���
�ӳɷ�Ӧ
�ӳɷ�Ӧ
��ȡ����Ӧ
ȡ����Ӧ
��������Ӧ
������Ӧ
0��2��д�����з���ʽ����
2CH3CH2OH+O2
2CH3CHO+2H2O
| Cu |
| �� |
2CH3CH2OH+O2
2CH3CHO+2H2O
��| Cu |
| �� |
CH3CH=CHCHO+2H2
CH3CH2CH2CH2OH
| ���� |
CH3CH=CHCHO+2H2
CH3CH2CH2CH2OH
��| ���� |


��3��д��D�����������Һ�����������ͬ���칹����
3
3
�֣�д������һ�ֺ˴Ź���������3���壬����ԭ����Ϊ6��1��1�ĽṹʽHCOOCH��CH3��CH3
HCOOCH��CH3��CH3
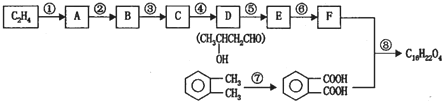
����������D��֪CΪCH3CHO����BΪCH3CH2OH��AӦΪCH3CH2Br��CH3CH2Cl��EΪCH3CH=CHCHO��FΪCH3CH2CH2CH2OH���� ����������Ӧ�����ɷ���ʽΪC18H22O4����������л���Ľṹ�������Լ���ĿҪ������⣮
����������Ӧ�����ɷ���ʽΪC18H22O4����������л���Ľṹ�������Լ���ĿҪ������⣮
 ����������Ӧ�����ɷ���ʽΪC18H22O4����������л���Ľṹ�������Լ���ĿҪ������⣮
����������Ӧ�����ɷ���ʽΪC18H22O4����������л���Ľṹ�������Լ���ĿҪ������⣮����⣺��D��֪CΪCH3CHO����BΪCH3CH2OH��AӦΪCH3CH2Br��CH3CH2Cl��EΪCH3CH=CHCHO��FΪCH3CH2CH2CH2OH���� ����������Ӧ�����ɷ���ʽΪC18H22O4������
����������Ӧ�����ɷ���ʽΪC18H22O4������
��1�������Ϸ�����Ϲ����ŵı仯��֪��Ϊ�ӳɷ�Ӧ����Ϊȡ����Ӧ����Ϊ������Ӧ���ʴ�Ϊ���ӳɷ�Ӧ��ȡ����Ӧ��������Ӧ��
��2����Ϊ�Ҵ���������Ӧ������ʽΪ2CH3CH2OH+O2
2CH3CHO+2H2O��
��ΪCH3CH=CHCHO�����������ӳɷ�Ӧ������ʽΪCH3CH=CHCHO+2H2
CH3CH2CH2CH2OH��
��CH3CH2CH2CH2OH�� ����������Ӧ������ʽΪ
����������Ӧ������ʽΪ ��
��
�ʴ�Ϊ��2CH3CH2OH+O2
2CH3CHO+2H2O��CH3CH=CHCHO+2H2
CH3CH2CH2CH2OH�� ��
��
��3��D�ķ���ʽΪC4H8O2����Ӧ�����������Һ�����������ͬ���칹����CH3CH2COOCH3��CH3COOCH2CH3��HCOOCH��CH3��CH3����3�֣�����һ�ֺ˴Ź���������3���壬����ԭ����Ϊ6��1��1�ĽṹʽΪHCOOCH��CH3��CH3��
�ʴ�Ϊ��3��HCOOCH��CH3��CH3��
 ����������Ӧ�����ɷ���ʽΪC18H22O4������
����������Ӧ�����ɷ���ʽΪC18H22O4��������1�������Ϸ�����Ϲ����ŵı仯��֪��Ϊ�ӳɷ�Ӧ����Ϊȡ����Ӧ����Ϊ������Ӧ���ʴ�Ϊ���ӳɷ�Ӧ��ȡ����Ӧ��������Ӧ��
��2����Ϊ�Ҵ���������Ӧ������ʽΪ2CH3CH2OH+O2
| Cu |
| �� |
��ΪCH3CH=CHCHO�����������ӳɷ�Ӧ������ʽΪCH3CH=CHCHO+2H2
| ���� |
��CH3CH2CH2CH2OH��
 ����������Ӧ������ʽΪ
����������Ӧ������ʽΪ ��
���ʴ�Ϊ��2CH3CH2OH+O2
| Cu |
| �� |
| ���� |
 ��
����3��D�ķ���ʽΪC4H8O2����Ӧ�����������Һ�����������ͬ���칹����CH3CH2COOCH3��CH3COOCH2CH3��HCOOCH��CH3��CH3����3�֣�����һ�ֺ˴Ź���������3���壬����ԭ����Ϊ6��1��1�ĽṹʽΪHCOOCH��CH3��CH3��
�ʴ�Ϊ��3��HCOOCH��CH3��CH3��
���������⿼���л�����ƶϣ�Ϊ�߿��������ͺ�Ƶ���㣬������ѧ���ķ��������Ŀ��飬ע�����D�Ľṹ�������Ϣ�ƶϣ���ȷ�л���Ľṹ��ʽ������Ϊ������Ĺؼ����Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ

 H2SiO3+2OH-����SiO32-+H2O
H2SiO3+2OH-����SiO32-+H2O HSiO3-+OH-��HSiO3-+H2O
HSiO3-+OH-��HSiO3-+H2O H2SiO3+OH-��AlO2-+H2O
H2SiO3+OH-��AlO2-+H2O Al��OH��3+OH-��������pHֵ������ƽ��������Ӧ�����ƶ�����pH����7��8ʱ��ʹ����ˮ����ȫ
Al��OH��3+OH-��������pHֵ������ƽ��������Ӧ�����ƶ�����pH����7��8ʱ��ʹ����ˮ����ȫ Cr2O72-+H2O
Cr2O72-+H2O