��Ŀ����
��2013?����һģ������������ʾ���ʱ仯�Ļ�ѧ��Ӧ����ȷ���ǣ�������
������A��������д�Ȼ�ѧ����ʽ��Ҫ������жϣ�
B����������������������Ӧ��Ӧ���������Ӻ����������ӵķ�Ӧ��û�а������ɣ�
C������ǿ����Һ�У����ɵ�������������������������ˮ�жϣ�
D�����ݵ����غ�������غ��жϣ�
B����������������������Ӧ��Ӧ���������Ӻ����������ӵķ�Ӧ��û�а������ɣ�
C������ǿ����Һ�У����ɵ�������������������������ˮ�жϣ�
D�����ݵ����غ�������غ��жϣ�
����⣺A���кͷ�Ӧ���Ȼ�ѧ����ʽ�У�������������ʵ�״̬����ȷ���Ȼ�ѧ����ʽΪ��H+��aq��+OH-��aq���TH2O��1����H=-57.3kJ?mol-1����A����
B�����������Һ����������������Һ��Ϲ��ȣ�������Ӧ�������������������ӷ�Ӧ����ˮ����ȷ�����ӷ���ʽ��H++OH-�TH2O����B����
C����ǿ����Һ��NaClO�����������ӡ�Fe��OH��3��Ӧ����Na2FeO4�����ƺ�ˮ�����ݻ��ϼ����������ƽ����Ӧ�����ӷ���ʽΪ��3ClO-+4OH-+2Fe��OH��3=3Cl-+5H2O+2FeO42-����C����
D����ϡ�����ữ��KMnO4��Һ��H2O2��Ӧ�������������ӡ�������ˮ�����ݻ��ϼ����������ƽ����Ӧ�����ӷ���ʽΪ��2MnO4-+6H++5H2O2=2Mn2++5O2��+8H2O����D��ȷ��
��ѡD��
B�����������Һ����������������Һ��Ϲ��ȣ�������Ӧ�������������������ӷ�Ӧ����ˮ����ȷ�����ӷ���ʽ��H++OH-�TH2O����B����
C����ǿ����Һ��NaClO�����������ӡ�Fe��OH��3��Ӧ����Na2FeO4�����ƺ�ˮ�����ݻ��ϼ����������ƽ����Ӧ�����ӷ���ʽΪ��3ClO-+4OH-+2Fe��OH��3=3Cl-+5H2O+2FeO42-����C����
D����ϡ�����ữ��KMnO4��Һ��H2O2��Ӧ�������������ӡ�������ˮ�����ݻ��ϼ����������ƽ����Ӧ�����ӷ���ʽΪ��2MnO4-+6H++5H2O2=2Mn2++5O2��+8H2O����D��ȷ��
��ѡD��
���������⿼�����ӷ�Ӧ����ʽ����д�жϣ���ȷ�����Ļ�ѧ��Ӧ�����ӷ�Ӧ��Ӧ������ѧʽ�����ʼ��ɽ����Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
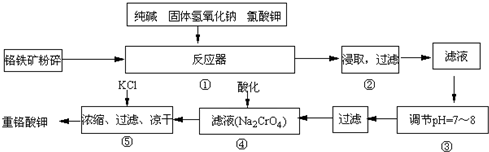
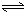 H2SiO3+2OH-����SiO32-+H2O
H2SiO3+2OH-����SiO32-+H2O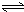 HSiO3-+OH-��HSiO3-+H2O
HSiO3-+OH-��HSiO3-+H2O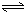 H2SiO3+OH-��AlO2-+H2O
H2SiO3+OH-��AlO2-+H2O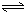 Al��OH��3+OH-��������pHֵ������ƽ��������Ӧ�����ƶ�����pH����7��8ʱ��ʹ����ˮ����ȫ
Al��OH��3+OH-��������pHֵ������ƽ��������Ӧ�����ƶ�����pH����7��8ʱ��ʹ����ˮ����ȫ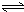 Cr2O72-+H2O
Cr2O72-+H2O