��Ŀ����
����Ŀ��ij��ȤС����ʵ����ģ�ҵ����Ԥ������ĸ������![]() ��Ҫ�ɷ�
��Ҫ�ɷ�![]() ��������
��������![]() Ϊԭ���Ʊ�
Ϊԭ���Ʊ�![]() ���ⶨ���ȣ��������ʵ�顣�ش��������⣺
���ⶨ���ȣ��������ʵ�顣�ش��������⣺
��![]() ���ո�����ۡ�
���ո�����ۡ�

��֪������ʱ��![]() ת��Ϊ
ת��Ϊ![]() ��
��
(1)ʢ��![]() ����������Ϊ________װ��B������Ϊ________��
����������Ϊ________װ��B������Ϊ________��
(2)![]() ����ѡ�ò���������ԭ��Ϊ________��
����ѡ�ò���������ԭ��Ϊ________��
(3)ʵ��ʱ��a�����ò����![]() ���һ�ֺ���ɫ���壬�ҹ۲쵽����ʯ��ˮ����ǣ���a�з���������ԭ��Ӧ�Ļ�ѧ����ʽΪ________��
���һ�ֺ���ɫ���壬�ҹ۲쵽����ʯ��ˮ����ǣ���a�з���������ԭ��Ӧ�Ļ�ѧ����ʽΪ________��
��![]() �Ʊ�
�Ʊ�![]() ��
��
(4)ʵ���������ȴ�����¡�ȡa�й����ˮ�ܽ⣬������Һ��pHΪ![]() �����ˣ�������Һ��
�����ˣ�������Һ��![]() ������Ũ�������½ᾧ�����ˡ�ϴ�ӣ���
������Ũ�������½ᾧ�����ˡ�ϴ�ӣ���![]() ��Ʒ��
��Ʒ��
�ٵ�����Һ��pHΪ![]() ��һ�ι��ˣ����ó����ijɷ�Ϊ________
��һ�ι��ˣ����ó����ijɷ�Ϊ________![]() �ѧʽ
�ѧʽ![]() ��
��
�ڹ���������Ҫ����������________��
�۽�����ӷ���ʽ˵��������Һ��![]() ��Ŀ��Ϊ________��
��Ŀ��Ϊ________��
��![]() �ⶨ��Ʒ��
�ⶨ��Ʒ��![]() ������������
������������
(5)��ȡ![]() ��Ʒ����������ˮ���ữ�����100mL��Һ��ȡ
��Ʒ����������ˮ���ữ�����100mL��Һ��ȡ![]() ������Һ���μӼ���ָʾ������
������Һ���μӼ���ָʾ������![]() ��
��![]() ��Һ�ζ����յ�
��Һ�ζ����յ�![]() ���ʲ��μӷ�Ӧ����ԭ����Ϊ
���ʲ��μӷ�Ӧ����ԭ����Ϊ![]() �����ı�Һ�����ΪVmL����Ʒ��
�����ı�Һ�����ΪVmL����Ʒ��![]() ����������Ϊ___
����������Ϊ___![]() �ú�c��m��V�Ĵ���ʽ��ʾ
�ú�c��m��V�Ĵ���ʽ��ʾ![]() ��
��
���𰸡�Բ����ƿ �������� ���������£������е�![]() ��
��![]() ��Ӧ 4Fe(CrO2)2+7O2+8K2CO3
��Ӧ 4Fe(CrO2)2+7O2+8K2CO3![]() 8K2CrO4+2Fe2O3+8CO2
8K2CrO4+2Fe2O3+8CO2 ![]() ��
��![]() �ձ���©���������� ʹ��ƽ�������ƶ���2CrO42-+2H+
�ձ���©���������� ʹ��ƽ�������ƶ���2CrO42-+2H+![]() Cr2O72-+H2O ����
Cr2O72-+H2O ����![]() �IJ���
�IJ��� ![]()
��������
��![]() ʢ��
ʢ��![]() ����������ΪԲ����ƿ��װ��B������Ϊ�����������ݴ˽��
����������ΪԲ����ƿ��װ��B������Ϊ�����������ݴ˽��
![]() ���������£������е�
���������£������е�![]() ��
��![]() ��Ӧ���ݴ˽��
��Ӧ���ݴ˽��
![]() ����Ϣ֪��
����Ϣ֪��![]() ��
��![]() ��
��![]() �����·�Ӧ����
�����·�Ӧ����![]() ��
��![]() ��
��![]() ���ݴ˽��
���ݴ˽��
��![]() ����Ϣ֪��������Һ��pHΪ
����Ϣ֪��������Һ��pHΪ![]() ��Ŀ���ǽ�
��Ŀ���ǽ�![]() ת��Ϊ
ת��Ϊ![]() ������
������![]() һ�����˳�ȥ���ݴ˽��
һ�����˳�ȥ���ݴ˽��
![]() ����������Ҫ�����������ձ���©�������������ݴ˽��
����������Ҫ�����������ձ���©�������������ݴ˽��
![]() ������Һ��
������Һ��![]() ����ʹ
����ʹ![]() ��ƽ�������ƶ����ݴ˽��
��ƽ�������ƶ����ݴ˽��
��![]() ���ݵ�ʧ�����غ�ã�
���ݵ�ʧ�����غ�ã�![]() ���ݴ˽��
���ݴ˽��
��![]() ʢ��
ʢ��![]() ����������ΪԲ����ƿ��װ��B������Ϊ�����������ʴ�Ϊ��Բ����ƿ������������
����������ΪԲ����ƿ��װ��B������Ϊ�����������ʴ�Ϊ��Բ����ƿ������������
![]() ���������£������е�
���������£������е�![]() ��
��![]() ��Ӧ����a�����ò����������ʴ�Ϊ�����������£������е�
��Ӧ����a�����ò����������ʴ�Ϊ�����������£������е�![]() ��
��![]() ��Ӧ��
��Ӧ��
![]() ����Ϣ֪��
����Ϣ֪��![]() ��
��![]() ��
��![]() �����·�Ӧ����
�����·�Ӧ����![]() ��
��![]() ��
��![]() ����ѧ����ʽΪ
����ѧ����ʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��![]() ����Ϣ֪��������Һ��pHΪ
����Ϣ֪��������Һ��pHΪ![]() ��Ŀ���ǽ�
��Ŀ���ǽ�![]() ת��Ϊ
ת��Ϊ![]() ������
������![]() һ�����˳�ȥ�������ó����ijɷ�Ϊ
һ�����˳�ȥ�������ó����ijɷ�Ϊ![]() ��
��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��![]() ��
��
![]() ����������Ҫ�����������ձ���©�������������ʴ�Ϊ���ձ���©������������
����������Ҫ�����������ձ���©�������������ʴ�Ϊ���ձ���©������������
![]() ������Һ��
������Һ��![]() ����ʹ
����ʹ![]() ��ƽ�������ƶ�������
��ƽ�������ƶ�������![]() �IJ��ʣ��ʴ�Ϊ��ʹ
�IJ��ʣ��ʴ�Ϊ��ʹ![]() ��ƽ�������ƶ�������
��ƽ�������ƶ�������![]() �IJ��ʣ�
�IJ��ʣ�
��![]() ���ݵ�ʧ�����غ�ã�
���ݵ�ʧ�����غ�ã�![]() �����Դ�Ʒ��
�����Դ�Ʒ��![]() ����������Ϊ
����������Ϊ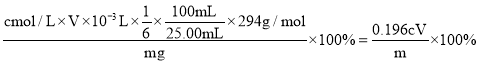 ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��

 ÿ�α���ϵ�д�
ÿ�α���ϵ�д� ��ѧ����ϵ�д�
��ѧ����ϵ�д�