��Ŀ����
4��������Ԫ��A��B��C��D�У�0.5mol AԪ�ص����ӵõ�6.02��1023�����ӱ���ԭΪ����ԭ�ӣ�0.4g A��������ǡ����100ml 0.2mol/L��������ȫ��Ӧ��Aԭ�Ӻ���������Ŀ��������Ŀ��ȣ�BԪ��ԭ�Ӻ���M�������Ŀ��K���1����C-��AԪ�ص����Ӷ�1�����Ӳ㣬DԪ�ص�ԭ�Ӻ���L���K���2�����ӣ���1��A��D��Ԫ�ط��ŷֱ���Mg��C��
��2������C-���ӵĽṹʾ��ͼ
 ��
����3��Ԫ��B�����ڱ���λ�ã��������ڵڢ�A�壮
��4����B��C����Ԫ���γɵĻ������ˮ��Һ����ε����������Һ������Ϊ��ʼ���ɰ�ɫ���������ɫ�����ܽ⣬д���й����ӷ���ʽAl3++3OH-�TAl��OH��3����Al��OH��3+OH-�TAlO2-+2H2O��
��5��д��A��C����Ԫ���γɵĻ�����ĵ���ʽ
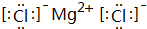 ��
��
���� ������Ԫ��A��B��C��D�У�0.5mol AԪ�ص����ӵõ�6.02��1023�����ӱ���ԭΪ����ԭ�ӣ���A����Ϊ��������λ����ɵ������ӣ�0.4g A��������ǡ����100ml 0.2mol/L��������ȫ��Ӧ����AO+2HCl�TACl2+H2O��M��AO��=$\frac{0.4g}{0.01mol}$=40g/mol������A��Ħ������Ϊ40g/mol-16g/mol=24g/mol����Aԭ�Ӻ���������Ŀ��������Ŀ��ȣ���������Ϊ12����AΪMgԪ�أ�BԪ��ԭ�Ӻ���M�������Ŀ��K���1������M�������Ϊ3����BΪAl��C-��A��Mg��Ԫ�ص����Ӷ�1�����Ӳ㣬��CΪClԪ�أ�DԪ�ص�ԭ�Ӻ���L���K���2�����ӣ�����L�������Ϊ4����DΪ̼Ԫ�أ��ݴ˽��
��� �⣺������Ԫ��A��B��C��D�У�0.5mol AԪ�ص����ӵõ�6.02��1023�����ӱ���ԭΪ����ԭ�ӣ���A����Ϊ��������λ����ɵ������ӣ�0.4g A��������ǡ����100ml 0.2mol/L��������ȫ��Ӧ����AO+2HCl�TACl2+H2O��M��AO��=$\frac{0.4g}{0.01mol}$=40g/mol������A��Ħ������Ϊ40g/mol-16g/mol=24g/mol����Aԭ�Ӻ���������Ŀ��������Ŀ��ȣ���������Ϊ12����AΪMgԪ�أ�BԪ��ԭ�Ӻ���M�������Ŀ��K���1������M�������Ϊ3����BΪAl��C-��A��Mg��Ԫ�ص����Ӷ�1�����Ӳ㣬��CΪClԪ�أ�DԪ�ص�ԭ�Ӻ���L���K���2�����ӣ�����L�������Ϊ4����DΪ̼Ԫ�أ�
��1��������������֪��A��D��Ԫ�ط��ŷֱ���Mg��C���ʴ�Ϊ��Mg��C��
��2��CԪ�ص�����ΪCl-�����ӽṹʾ��ͼΪ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��3��BΪAlԪ�أ����ڵ������ڢ�A�壬�ʴ�Ϊ��������A��
��4��B��C����Ԫ���γɵĻ�����ΪAlCl3����AlCl3��ˮ��Һ����ε����������Һ���й����ӷ���ʽ��Al3++3OH-�TAl��OH��3����Al��OH��3+OH-�TAlO2-+2H2O������Ϊ����ʼ���ɰ�ɫ���������ɫ�����ܽ⣬
�ʴ�Ϊ����ʼ���ɰ�ɫ���������ɫ�����ܽ⣻Al3++3OH-�TAl��OH��3����Al��OH��3+OH-�TAlO2-+2H2O��
��5��A��C����Ԫ���γɵĻ�����ΪMgCl2�������ʽΪ��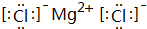 ��
��
�ʴ�Ϊ��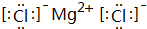 ��
��
���� ���⿼��ṹ����λ�ù�ϵӦ�ã��ƶ�Ԫ���ǽ���ؼ�����Ҫѧ����������Ԫ�ػ�����֪ʶ���ѶȲ���

| A�� | 1mol�κ����ʾ�����Լ6.02��1023������ | |
| B�� | 0.012kg̼-12�к���Լ6.02��1023��̼ԭ�� | |
| C�� | 1molˮ�к���2 mol���1mol�� | |
| D�� | 1mol H2����6.02��1023������ |
| ʵ�鷽�� | ʵ�鷽�� | �������� | |
| A�� | ������ | ����Ʒ�����ᷴӦ������ͨ����ˮ����װ������ | ��ˮ��� |
| B�� | ������ | ����Ʒ�����ᷴӦ�����ɵ�����ȫ������ʯ������ | ��ʯ������ |
| C�� | ������ | ��Ʒ������ƿ�У����ڵ�����ƽ�ϣ������������� | ��������� |
| D�� | �ζ��� | ����Ʒ���100mL��Һ��ȡ10mL�����̪���ñ�����ȷ�� | ������������ |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ͨ��������ˮ�� | B�� | �ڿ�����ȼ�� | ||
| C�� | ͨ�����Ը��������Һ�� | D�� | ͨ��Һ���� |
 ʵ�����Ʊ��������IJ������£�
ʵ�����Ʊ��������IJ������£�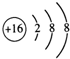 ��
��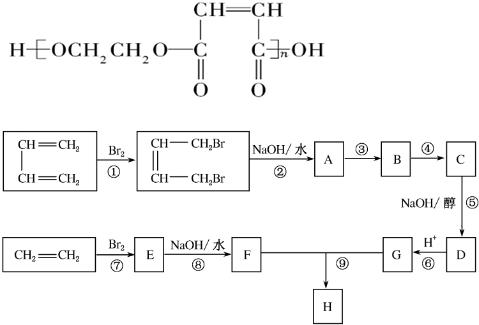
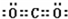 ��Vԭ�ӵĽṹʾ��ͼ
��Vԭ�ӵĽṹʾ��ͼ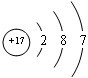 ��Z���⻯��ṹʽΪ
��Z���⻯��ṹʽΪ ��д��UԪ�������ڱ��е�λ�õ������ڵ�VIA��N2W2�к��еĻ�ѧ�����������Ӽ������ۼ�
��д��UԪ�������ڱ��е�λ�õ������ڵ�VIA��N2W2�к��еĻ�ѧ�����������Ӽ������ۼ�