��Ŀ����
�������⡿�����仯�����������������Ӧ�ù㷺��
��1��N2O��һ��ǿ����������壬��һ�������£���ֽ�Ļ�ѧ����ʽΪ��2N2O(g)=2N2(g)+O2(g) ��H<0������Ϊ�÷�Ӧ���淴Ӧ�Ƿ��Է����У����жϲ�˵������___________��
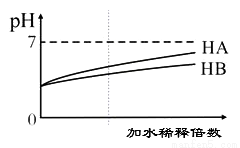
ij�о�С��Ϊ���N2O�ķֽ�����о��������£���1L���������г���0.100mol N2O���壬���ȵ�һ���¶Ⱥ�ʹ֮��Ӧ���뻭���������������Ũ��c(��)��ʱ��t�仯��������ͼ��_____________

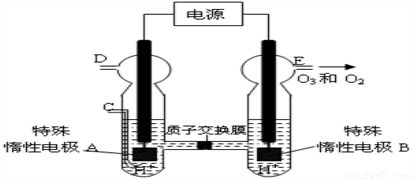
��2����N2O5��һ����������������ҵ�Ͽ���N2O4ͨ������Ʊ�N2O5������������֮�������ӽ���Ĥ�������������Һ�ֱ�Ϊϡ������Һ������N2O4����ˮ���ᣬ�ö��Ե缫���ʱ������N2O5���ҵĵ缫��ӦʽΪ_____________��
����֪��һ�������������з�Ӧ��
��2N2O5(g)  4NO2 (g)+ O2(g) ��H1
4NO2 (g)+ O2(g) ��H1
��2NO2(g)  2NO (g)+ O2(g) ��H2
2NO (g)+ O2(g) ��H2
��2NaOH(aq)+N2O5(g)=2NaNO3(aq)+H2O(1) ��H3
д��NO��O2�Ļ��������NaOH��Һ���������ε��Ȼ�ѧ����ʽ___________��
���ݻ�Ϊ2L�ĺ����ܱ������г���1.00mol N2O5��������T��ʱֻ������Ӧ�����ﵽƽ��״̬��O2Ϊ1.00mol��NO2Ϊ0.400mol,��T��ʱ��Ӧ���ƽ�ⳣ����ֵΪ_________��
��3��NH3��Cr2O3�����ڸ����¿����Ʊ���ܲ���CrN���壬�䷴ӦΪCr2O3(s)+2NH3(g)  2CrN(s)+3H2O(g)����800��ʱ��Ϊ������ͬʱ�����ռ����Ĵֲ�Ʒ�����ĺ�����ͨ����߷�Ӧ����NH3(g)�����Ĺ����ֶδ�ɣ�����Ϳ��ܵ�ԭ��___________��
2CrN(s)+3H2O(g)����800��ʱ��Ϊ������ͬʱ�����ռ����Ĵֲ�Ʒ�����ĺ�����ͨ����߷�Ӧ����NH3(g)�����Ĺ����ֶδ�ɣ�����Ϳ��ܵ�ԭ��___________��
 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�



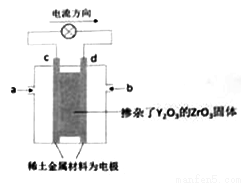
 O2(g)===CO(g)����H��w��
O2(g)===CO(g)����H��w��