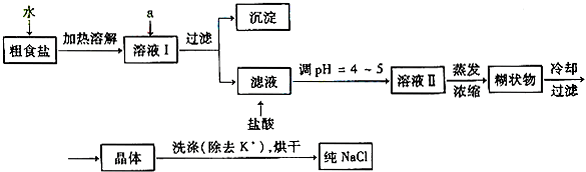
��Ŀ����
��13�֣�ʳ�����ճ�����ı���Ʒ��Ҳ����Ҫ�Ļ���ԭ�ϡ�
�Ŵ�ʳ�γ���������K+��Ca2+��Mg2+��Fe3+��SO42-���������ӣ�ʵ�����ᴿNaCl���������£�
�ṩ���Լ�������Na2CO3��Һ ����K2CO3��Һ NaOH��Һ BaCl2��Һ Ba(NO3)2��Һ ���Ȼ�̼
����ȥ��Һ���е�Ca2+��Mg2+��Fe3+��SO42-���ӣ�ѡ��a���������Լ������μ�˳������Ϊ ����ֻ�ѧʽ��
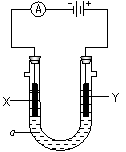 �����ᴿ��NaCl����500mL4.00 mol?L-1NaCl��Һ������������ҩ�ס����������
�����ᴿ��NaCl����500mL4.00 mol?L-1NaCl��Һ������������ҩ�ס����������
�����������ƣ���
�ǵ�ⱥ��ʳ��ˮ��װ����ͼ��ʾ������ڱ���ʳ��ˮ�еμӷ�̪��ͨ��� (��X��Y)��������Һ��죬д����ⱥ��ʳ��ˮ�Ļ�ѧ����ʽ ��
��ʵ�����Ʊ�H2��Cl2ͨ���������з�Ӧ��
Zn��H2SO4====ZnSO4��H2�� MnO2��4HCl(Ũ)![]() MnCl2��Cl2����2H2O
MnCl2��Cl2����2H2O
�ݴˣ���������������װ����ѡ���Ʊ����ռ�H2��װ�� ������ţ����Ʊ����ռ��������Cl2��װ�� ������ţ���
��ѡ���Ʊ������װ�ã� 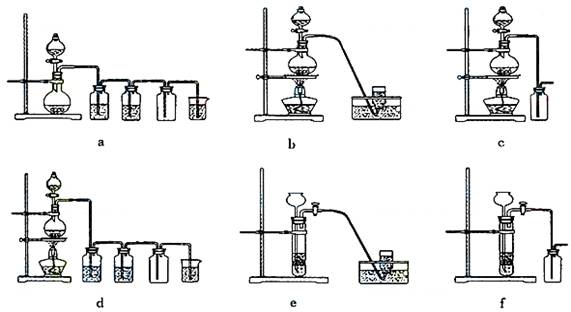
��1��NaOH��BaCl2��Na2CO3��BaCl2��NaOH��Na2CO3�� ��2�֣�
����ѡ����ѡ�����֡�NaOH��Һ�ļ���˳���Ƿ��NaOH��Һ��Ӱ��÷֣�
��2����ƽ���ձ���500mL����ƿ����ͷ�ι�(4��)����һ����1�֣�д��1����1�֣�����Ϊֹ��
��3�� X ��1�֣�
![]() ��2�֣�
��2�֣�
��4�� e ��2�֣� d ��2�֣�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�