��Ŀ����
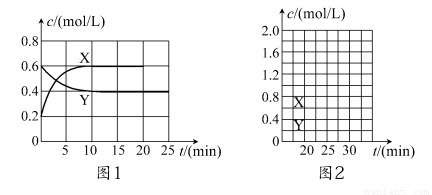
��һ����NO2��N2O4�Ļ������ͨ�����Ϊ2 L�ĺ����ܱ������У�������Ũ����ʱ��仯�Ĺ�ϵ��ͼ1��ʾ��
��ش�
(1)ͼl�У�����________(����X������Y��)��ʾNO2Ũ����ʱ��ı仯�����ǰ10 min��v(NO2)��________mol/(L��min)��
(2)����ѡ���в���˵���÷�Ӧ�Ѵﵽƽ��״̬����________(��ѡ����ĸ)��
A�������ڻ�������ѹǿ����ʱ��仯���ı�
B�������ڻ��������ܶȲ���ʱ��仯���ı�
C�������ڻ���������ɫ����ʱ��仯���ı�
D�������ڻ�������ƽ����Է�����������ʱ��仯���仯
(3)��Ӧ���е�10 minʱ������������22.76 kJ����÷�Ӧ���Ȼ�ѧ����ʽΪ_________________________________���÷�Ӧ��ƽ�ⳣ��K��________��
(4)��Ӧ���е�20 minʱ�����������ڳ���һ����NO2,10 min��ﵽ�µ�ƽ�⣬��ʱ���c(NO2)��0.9 mol/L��
����һ��ƽ��ʱ���������NO2���������Ϊ��1���ﵽ��ƽ�����������NO2���������Ϊ��2������1________��2(����>��������������<��)��
������ͼ2�л���20 min������ʵ�Ũ����ʱ��仯������(�����ϱ�������X������Y��)��
(1)X��0.04��(2)B
(3)N2O4(g)  2NO(g)����H����56.9kJ/mol��0.9
2NO(g)����H����56.9kJ/mol��0.9
(4)��>����
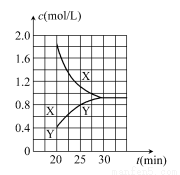
ע��ÿ�����߰�����㡢�յ㡢��X������Y����ע�ȡ�
��������(1)��ͬʱ����X��Y��Ũ�ȱ仯��֮��Ϊ2��1����XӦ��ʾNO2������v�� ��֪��v(NO2)��
��֪��v(NO2)��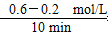 ��0.04 mol/(L��min)��
��0.04 mol/(L��min)��
(2)���ڷ�ӦN2O4(g)??2NO2(g)�����������£�ѹǿ����ɫ�������ƽ����Է�������(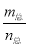 )��Ϊ�������ܶ�(
)��Ϊ�������ܶ�(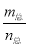 )�Ƕ�ֵ����A��C��D����˵���÷�Ӧ�Ѵ�ƽ�⣬ֻ��B����˵����
)�Ƕ�ֵ����A��C��D����˵���÷�Ӧ�Ѵ�ƽ�⣬ֻ��B����˵����
(3)��Ӧ���е�10 minʱN2O4����0.4 mol������22.76 kJ��������Ӧ����1 mol N2O4ʱ������56.9 kJ������ƽ�ⳣ��K��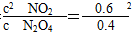 ��0.9��
��0.9��
(4)ƽ������������ڳ���һ����NO2���൱������ƽ����ϵ��ѹǿ����ʹ��ӦN2O4(g)  2NO2(g)��ƽ�����ƣ��¶Ȳ��䣬ƽ�ⳣ�����䣬��0.9��
2NO2(g)��ƽ�����ƣ��¶Ȳ��䣬ƽ�ⳣ�����䣬��0.9��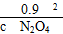 �����ﵽ��ƽ��ʱc(N2O4)��0.9 mol/L���ڶ���ƽ��ʱNO2�����������2��
�����ﵽ��ƽ��ʱc(N2O4)��0.9 mol/L���ڶ���ƽ��ʱNO2�����������2��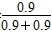 ��100%��50%����һ��ƽ��ʱNO2�����������1��
��100%��50%����һ��ƽ��ʱNO2�����������1��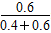 ��100%��60%���ɴ˵ó���1>��2��
��100%��60%���ɴ˵ó���1>��2��

 ���������������Բ��������ϵ�д�
���������������Բ��������ϵ�д�