��Ŀ����
2013��ȫ�����ض�������ʮ�������������У�������β����ȼú�����������Կ����������ϴ�
��1������β����������Ҫԭ��Ϊ��2NO(g) + 2CO(g)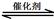 2CO2(g)+ N2(g)����H��0
2CO2(g)+ N2(g)����H��0
���÷�Ӧ�ھ��ȡ����ݵ��ܱ���ϵ�н��У�����ʾ��ͼ��ȷ����˵����Ӧ�ڽ��е�t1ʱ�̴ﵽƽ��״̬���� ������ţ���
����ͼ�Ц�����K��n��w�ֱ��ʾ����Ӧ���ʡ�ƽ�ⳣ�������ʵ���������������
��2��������β����úȼ�ղ����������������������CH4����ԭNOX�������������������Ⱦ����֪��CH4(g)+2NO2(g)��N2(g)��CO2(g)+2H2O(g) ��H����867 kJ/mol
2NO2(g)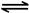 N2O4(g) ��H����56.9 kJ/mol
N2O4(g) ��H����56.9 kJ/mol
H2O(g) �� H2O(l) ��H �� ��44.0 kJ��mol
д��CH4����ԭN2O4(g)����N2��H2O(l)���Ȼ�ѧ����ʽ�� ��
��3����NH3����ԭNOXҲ�������������������Ⱦ����ͼ������NH3����ԭ����������һ��������ͨ�����ֲ�ͬ�����������ݳ������е������ﺬ�����Ӷ�ȷ�������ѵ��ʣ�ע���ѵ��ʼ���������ת���ʣ���
��Ӧԭ��Ϊ��NO(g) +NO2(g)+2NH3(g)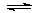 2N2(g) + 3H2O(g)��
2N2(g) + 3H2O(g)��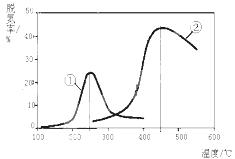
�ٸ÷�Ӧ�ġ�S 0����H 0����������������� ����������
�ڶ������巴Ӧ����ij���(B)��ƽ��ѹǿ(pB)�������ʵ���Ũ��(cB)Ҳ���Ա�ʾƽ�ⳣ��������KP����
��������Ӧ��KP�� ��
������˵����ȷ���� ��
A���ڢ��ִ����ȵڢ��ִ����ѵ��ʸ�
B����ͬ�����£��ı�ѹǿ���ѵ���û��Ӱ��
C�������١��ڷֱ��ʺ���250���450�������ѵ�
��4��NO2��O2������NaNO3������ȼ�ϵ�أ���ԭ����ͼ���õ����ʹ�ù�����ʯīI�缫������������Y����缫��ӦΪ ��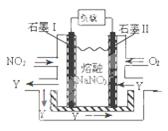
��5�����Ṥҵβ���е������NO��NO2���������ء�CO(NH2)2����Һ��ȥ����Ӧ���ɶԴ�������Ⱦ�����塣1 mol���������չ�ҵβ���е����������NO��NO2�����Ϊ1:1��������Ϊ___________g��
��1�� bd ����1�֣���2�֣�ѡ�����÷֣�
��2��CH4(g)+N2O4(g) =N2(g) +2H2O(g) + CO2(l) ��H=" ��898.1kJ/mol" ��2�֣�
��3���� ����1�֣�������1�֣���KP�� ��2�֣� �� �� C��2�֣�
��2�֣� �� �� C��2�֣�
��4��NO2+NO3�� ��e-=N2O5 ��2�֣�
��5��76g��2�֣�
���������������1��a������Ӧ���ʵ����淴Ӧ����ʱ���ﵽƽ��״̬����ʱ��Ӧ���ʲ��ٱ仯������ͼ���ϣ�����b����Ϊ����Ϊ���ȣ�����t1ʱƽ�ⳣ�����䣬˵����Ӧ�Ѵﵽƽ�⣬��ȷ��c��t1ʱCO��CO2�����ʵ������ڱ仯��û�дﵽƽ��״̬������d��t1ʱNO�������������ٱ仯��˵����Ӧ�Ѵﵽƽ�⣬��ȷ��
��2������д����ѧ����ʽ��ע��״̬��Ȼ����ݸ�˹��������ʱ䣬��H=?H1-?H2+2��?H3= ��898.1kJ?mol?1����д���Ȼ�ѧ����ʽ��
��3���١����ݻ�ѧ����ʽ��֪�����ϵ������˵��?S>0���Ѱ��ʴﵽ��ߵ�֮����������¶ȣ��Ѱ��ʽ��ͣ�˵��ƽ�����淴Ӧ�����ƶ���������ӦΪ���ȷ�Ӧ��?H<0��
�ڡ����ݻ�ѧ����ʽ�����ʵ�״̬��ϵ���ɵã�KP��
��A�����ִ����ڱ����¶�ʱ�ѵ��ʲ�ͬ������B���ı�ѹǿ����ѧƽ���ƶ����ѵ��ʷ����仯������
C����������250��ʱ�ѵ�����ߣ���������450�������ѵ�����ߣ���ȷ��
��4��ȼ�ϵ����O2�������Ϸ����õ��ӷ�Ӧ����NO2�ڸ�����ʧ�������ɸ���̬��N2O5���缫����ʽΪ��NO2+NO3�� ��e-= N2O5
��5����������NO��NO2���ɶԴ�������Ⱦ�����壬��ѧ����ʽΪ��CO(NH2)2 + NO +NO2 =2N2 +CO2+2H2O��1mol���ط�Ӧ����1mol NO��1mol NO2������Ϊ��30g+46g=76g��
���㣺���⿼�黯ѧƽ����жϡ��Ȼ�ѧ����ʽ���˹���ɡ�?H��?S���жϡ���ѧƽ�ⳣ������ѧƽ����жϡ�ȼ�ϵ�ء���ѧ���㡣

�״���һ�ֿ�������Դ�����й㷺�Ŀ�����Ӧ��ǰ����
(1)��ҵ��һ������������ַ�Ӧ�ϳɼ״���
��Ӧ��CO(g)��2H2(g) CH3OH(g)����H1
CH3OH(g)����H1
��Ӧ��CO2(g)��3H2(g) CH3OH(g)��H2O(g)����H2
CH3OH(g)��H2O(g)����H2
��������Ӧ���ϡ�ԭ�Ӿ��á�ԭ�����________(���)��
���±����������Ƿ�Ӧ���ڲ�ͬ�¶��µĻ�ѧƽ�ⳣ��(K)��
| �¶� | 250 �� | 300 �� | 350 �� |
| K | 2.041 | 0.270 | 0.012 |
�ɱ��������жϣ���H1______0(�����������������)��
��ij�¶��£���2 mol CO��6 mol H2����2 L���ܱ������У���ַ�Ӧ���ﵽƽ����c(CO)��0.2 mol��L��1����CO��ת����Ϊ________����ʱ���¶�Ϊ________(���ϱ���ѡ��)��
(2)��֪�ڳ��³�ѹ�£�
��2CH3OH(l)��3O2(g)===2CO2(g)��4H2O(g) ��H1����1 275.6 kJ��mol��1
��2CO(g)��O2(g)===2CO2(g) ��H2����566.0 kJ��mol��1
��H2O(g)===H2O(l)����H3����44.0 kJ��mol��1
д���״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽ��__________��
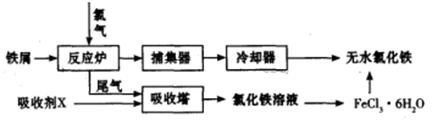
�ҹ��Ǹ��������,��������Ϊ�����һ,��¯��������Ϊ�ձ������������
I.��֪��Ӧ 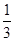 Fe2O3(s)+ CO(g)
Fe2O3(s)+ CO(g)
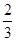 Fe(s)+ CO2(g) ��H��-23.5 kJ��mol-1���÷�Ӧ��
Fe(s)+ CO2(g) ��H��-23.5 kJ��mol-1���÷�Ӧ��
1000���ƽ�ⳣ������4����һ���ݻ�Ϊ10L���ܱ�������,1000��ʱ����Fe��Fe2O3��CO��CO2��1. 0mol,��Ӧ����l0min��ﵽƽ�⡣
��1��CO��ƽ��ת����=____________
��2�������CO��ƽ��ת����,�ٽ�Fe2O3��ת��,�ɲ�ȡ�Ĵ�ʩ��________
a����߷�Ӧ�¶�
b������Ӧ��ϵ��ѹǿ
c��ѡȡ���ʵĴ���
d����ʱ���ջ��Ƴ�����CO2
e�������ʯ,ʹ����ƽ���������ֽӴ�
��.��¯���������ķ����е�CO�ɽ��л���,ʹ����һ�������º�H2��Ӧ�Ʊ��״�:
CO(g)+ 2H2(g) CH3OH(g)�������ͼʾ�ش���������:
CH3OH(g)�������ͼʾ�ش���������: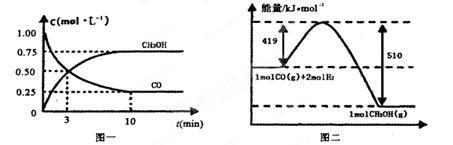
��1���ӷ�Ӧ��ʼ��ƽ��,��H2Ũ�ȱ仯��ʾƽ����Ӧ����v(H2)=________
��2�������¶Ⱥ�������ͬ�������ܱ�������,����ͬ��ʽͶ�뷴Ӧ��,��÷�Ӧ�ﵽƽ�ⅼ���й��������±���
| ���� | ��Ӧ��Ͷ����� | ��Ӧ��� ת���� | CH3OH��Ũ�� | �����仯 (Q1��Q2��Q3������0) |
| �� | 1mol CO��2mol H2 | ��1 | c1 | �ų�Q1kJ���� |
| �� | 1mol CH3OH | ��2 | c2 | ����Q2kJ���� |
| �� | 2mol CO��4mol H2 | ��3 | c3 | �ų�Q3kJ���� |
�����й�ϵ��ȷ����________
A��c1=c2 B��2Q1=Q3 C��2��1=��3 D����1+��2 =1
E���÷�Ӧ������1mol CH3OH����ų�(Q1+Q2)kJ����
���Լ���Ϊȼ�ϵ����͵�أ���ɱ�����������Ϊȼ�ϵĴ�ͳȼ�ϵ�أ�Ŀǰ�õ��㷺���о�����ͼ��Ŀǰ�о��϶��һ�����������ȼ�ϵ�ع���ԭ��ʾ��ͼ���ش��������⣺
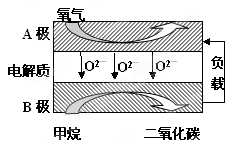
��1��B���ϵĵ缫��ӦʽΪ
��2�����ø�ȼ�ϵ������Դ����ʯī���缫���100mL 1mol/L������ͭ��Һ���������ռ���������������ʱ�����������ĵļ�������Ϊ ������£���
��ͼ��ú������ҵ����һ���֣���������ѧ֪ʶ������������⣺ 
I����֪�ò�ҵ����ij��Ӧ��ƽ���������ʽΪ��K�� ��������Ӧ��Ӧ�Ļ�ѧ����ʽΪ ��
��������Ӧ��Ӧ�Ļ�ѧ����ʽΪ ��
II�������ѣ�CH3OCH3����δ������������ͺ�Һ������Ϊ�ྻҺ��ȼ��ʹ�ã���ҵ����CO��H2Ϊԭ������CH3OCH3����ҵ�Ʊ��������ڴ���Ӧ���У�ѹ��2��0��10��0Mpa���¶�230��280�棩�������з�Ӧ��
��CO(g)��2H2(g) CH3OH(g) ��H1����90��7kJ��mol��1
CH3OH(g) ��H1����90��7kJ��mol��1
��2CH3OH(g) CH3OCH3(g)��H2O(g) ��H2����23��5kJ��mol��1
CH3OCH3(g)��H2O(g) ��H2����23��5kJ��mol��1
��CO(g)��H2O(g) CO2(g)��H2(g) ��H3����41��2kJ��mol��1
CO2(g)��H2(g) ��H3����41��2kJ��mol��1
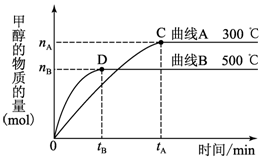
��1������Ӧ�����ܷ�Ӧ���Ȼ�ѧ����ʽΪ ��
830��ʱ��Ӧ�۵�K��1��0�����ڴ���Ӧ���з�Ӧ�۵�K 1��0�����������������������
��2����ij�¶��£�����Ӧ�ٵ���ʼŨ�ȷֱ�Ϊ��c(CO)��1 mol/L��c(H2)��2��4 mol/L��5 min��ﵽƽ�⣬CO��ת����Ϊ50������5 min��CO��ƽ����Ӧ����Ϊ �� ������Ӧ�����ʼŨ�ȷֱ�Ϊ��c(CO)��4 mol/L��c(H2)��a mol/L���ﵽƽ���c(CH3OH)��2 mol/L��a�� mol/L��
��3����Ӧ����t��ʱ��ƽ�ⳣ��Ϊ400�����¶��£���0��5L���ܱ������м���һ���ļ״�����Ӧ��ijʱ�̲�ø���ֵ����ʵ���Ũ�����£�
| ���� | CH3OH | CH3OCH3 | H2O |
| c(mol/L) | 0��8 | 1��24 | 1��24 |
�ٴ�ʱ��v�� v��������ڡ���С�ڡ����ڡ�
��ƽ��ʱ�����ѵ����ʵ���Ũ���� ��
�Զ����ѡ�������KOH ��ҺΪԭ�ϣ���ʯīΪ�缫��ֱ�ӹ���ȼ�ϵ�أ���õ�صĸ�����ӦʽΪ ������1��12L/min����״����������������ͨ������ѣ��øõ�ص��500mL2mol/L CuSO4��Һ��ͨ��0��50 min���������Ͽ���������ͭ������Ϊ
 Fe3��(aq)��3OH��(aq) ��H��a kJ/mol
Fe3��(aq)��3OH��(aq) ��H��a kJ/mol O2(g)===
O2(g)=== P4O10(s) ��H2����738.5 kJ��mol��1
P4O10(s) ��H2����738.5 kJ��mol��1
 CH3OH ( g ) ��H��-90.8 kJ��mol��1 ��һ�ݻ��ɱ���ܱ������г���10 mol CO ��20 molH2��CO ��ƽ��ת�������¶ȣ�T����ѹǿ��P���ı仯��ͼ��ʾ�����ﵽƽ��״̬A ʱ�����������Ϊ20 L��
CH3OH ( g ) ��H��-90.8 kJ��mol��1 ��һ�ݻ��ɱ���ܱ������г���10 mol CO ��20 molH2��CO ��ƽ��ת�������¶ȣ�T����ѹǿ��P���ı仯��ͼ��ʾ�����ﵽƽ��״̬A ʱ�����������Ϊ20 L��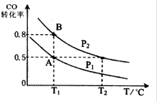
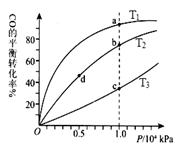
 CO��g��+H2O��g�� ��H=" +" 41.3 kJ��mol��1�������
CO��g��+H2O��g�� ��H=" +" 41.3 kJ��mol��1�������