题目内容
【题目】某次实验需用0.6mol·L-1 NaOH溶液480 mL。配制方法如下:
(1)配制该溶液应选用_______________mL容量瓶;
(2)用托盘天平准确称量__________g 固体NaOH;
(3)将称量好的NaOH固体放在500 mL大烧杯中,倒入约200 mL蒸馏水,用玻璃棒搅拌,使固体全部溶解,待__________________后,将烧杯中的溶液注入容量瓶中;
(4)用少量蒸馏水洗涤烧杯___________次,洗涤后的溶液___________________,轻轻晃动容量瓶,使溶液混合均匀;
(5)向容量瓶中加入蒸馏水,到液面______________时,改用___________加蒸馏水至液面最低点与刻度线相切。盖好瓶塞,______________;
(6)若在配制过程中出现下列情况,将使所配制的NaOH溶液的浓度偏高的是__________,偏低的是__________,对实验结果没有影响的是_______(填各选项的序号)。
A.所用的NaOH中混有少量Na2CO3
B.用托盘天平称量一定质量固体NaOH时,所用的小烧杯内壁不太干燥
C.配制溶液所用的容量瓶洗净后没有烘干
D.固体NaOH在烧杯中溶解后,立即将溶液转移到容量瓶内并接着进行后续操作
E.转移溶液后,未洗涤烧杯和玻璃棒就直接定容
F.最后确定NaOH溶液体积(定容)时,俯视观察液面与容量瓶刻度线
G.定容摇匀后静止,发现液面低于刻度线,再加蒸馏水至刻度线。
【答案】500 12.0 冷却到室温 2~3 注入容量瓶中 离刻度线1~2cm 胶头滴管 上下颠倒,摇匀 DF ABEG C
【解析】
(1)一定容积的容量瓶只能配制相应体积的溶液,根据溶液的体积480mL,但容量瓶的规格没有480mL,容量瓶体积要大于480mL且相近;
(2)利用n=c·V计算出氢氧化钠的物质的量,再根据m=n·M计算所需氢氧化钠的质量;
(3)氢氧化钠溶解放出大量的热,溶液具有热胀冷缩的性质,应先冷却至室温再移液定容;
(4)为保证溶质尽可能转移到容量瓶中,需用少量蒸馏水洗涤烧杯2-3 次,并将洗涤液一起转入容量瓶中;
(6)向容量瓶中加入蒸馏水,到液面 1-2cm时,改用胶头滴管加蒸馏水至液面最低点与刻度线相切;
(6)分析操作对溶质的物质的量或对溶液的体积的影响,根据c=![]() 分析判断。
分析判断。
(1)因溶液的体积480mL,但容量瓶的规格没有480mL,根据选择仪器的标准“大而近”的原则,要选用500mL容量瓶;
(2)需氢氧化钠的质量为m=0.5L×0.6molL-1×40g/mol=12.0g;
(3)氢氧化钠溶解放出大量的热,溶液具有热胀冷缩的性质,影响溶液体积,容量瓶要求使用的温度是20℃,属于应先NaOH溶液冷却至室温后再转移至500mL的容量瓶中;
(4)为保证溶质尽可能转移到容量瓶中,需用少量蒸馏水洗涤烧杯2-3次,并将洗涤液一起转入容量瓶中;
(5)向容量瓶中加入蒸馏水,到液面离刻度线下1-2cm时,改用胶头滴管加蒸馏水至液面最低点与刻度线相切,盖好瓶塞,上下颠倒,摇匀;
(6)A.所用的NaOH中混有少量Na2CO3,则等质量的样品中含有的NaOH的物质的量偏少,则根据c=![]() 可知:溶液的浓度偏低;
可知:溶液的浓度偏低;
B.用托盘天平称量一定质量固体NaOH时,所用的小烧杯内壁不太干燥,导致称取的氢氧化钠固体的物质的量偏小,导致溶液浓度偏低;
C.因定容时需向容量瓶中加水,所以定容前配制溶液所用的容量瓶洗净后没有烘干,对溶液浓度无影响;
D.液体具有热胀冷缩的性质,氢氧化钠溶解放热,未冷却到室温,趁热将溶液到入容量瓶,并配成了溶液,冷却至室温时溶液的体积变小,由于溶质的物质的量不变,因此会导致溶液浓度偏高;
E.转移溶液后,未洗涤烧杯和玻璃棒就直接定容,少量溶质氢氧化钠会沾在烧杯壁与玻璃棒上,导致氢氧化钠的实际质量减小,最终溶液浓度偏低;
F.定容时,俯视容量瓶刻度线,使溶液的体积偏低,最终使溶液浓度偏高;
G.定容摇匀后静止,液面低于刻度线,是由于一部分溶液留在瓶塞与瓶口之间,再加蒸馏水至刻度线,导致溶液体积偏大,最终使溶液浓度偏低。
因此最终使溶液的浓度偏高的有DF,使溶液的浓度偏低有ABEG,无影响的是B。

【题目】(1)了解用药常识,有利于自我保健。现有下列药物:
A.阿司匹林(乙酰水杨酸) B.青霉素 C.抗酸药(氢氧化铝) D.麻黄碱
①可治疗支气管哮喘,但不宜过多服用的药物是____________。(填字母)
②治疗胃酸过多,但患有严重的胃溃疡,应该选择___________。(填字母)
③一种重要的抗生素类药,有阻止多种细菌生长的功能,该药物是____________。(填字母)
④能使发热的病人体温降至正常,并起到缓解疼痛的作用,该药物是___________。(填字母)
(2)防治环境污染,改善生态环境已成为全球的共识。
①今年入冬以来,全国多个省市出现严重的雾霾天气。导致雾霾形成的主要污染物是_______(填字母)。
A.SO2 B.NO2 C.PM2.5
②垃圾应分类收集。导致“白色污染”的生活垃圾应放置于贴有________ (填字母)标志的垃圾筒内。

③工业废水需处理达标后才能排放。下列废水处理的方法合理的是____________。
A.用中和法除去废水中的酸
B.用混凝剂除去废水中的重金属离子
C.用氯气除去废水中的悬浮物
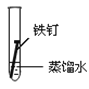
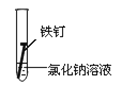
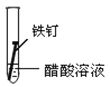
(3)全世界每年因钢铁锈蚀造成大量的损失。某学生欲探究在蒸馏水、氯化钠溶液和醋酸溶液三种条件下铁锈蚀的快慢,设计了如下实验。
实验序号 | Ⅰ | Ⅱ | Ⅲ |
实验内容 |
|
|
|
请回答:
①在一周的观察过程中,他发现实验序号为______的试管中铁钉锈蚀速度最慢。
②下列防止钢铁锈蚀的措施不合理的是__________(填字母)。
A.在自行车的钢圈表面镀镍
B.在地下钢铁管道上连接铜块
C.改变金属内部结构制成不锈钢
③炒过菜的铁锅未及时洗净(残液中含NaCl),第二天便会因腐蚀出现红褐色锈斑。试回答:铁锅的腐蚀主要是由__________________腐蚀造成的。