��Ŀ����
19�����屽��ϩ���ϩ�Ĺ�������һ�ָ߷�����ȼ�������еͶ������ȶ��Ժõ��ŵ㣮���������գ�
��1��д���ù�����Ľṹ��ʽ��
 ��
��
��2��ʵ�������ұ���ȡ���屽��ϩ�����Ⱦ�������Ӧ�Ƶ��м���
 ��д����������Ӧ������Լ�������Һ�塢�廯�����������塢����
��д����������Ӧ������Լ�������Һ�塢�廯�����������塢������3����
 ����������������Һ���ȵõ�A��A��������������FeCl3��Һ����ɫ��
����������������Һ���ȵõ�A��A��������������FeCl3��Һ����ɫ��A�Ľṹ��ʽΪ
 ��
����������Ӧ����֪����A���ɶ��屽��ϩ�ķ�Ӧ����ΪŨ���ᡢ���ȣ�
��4����ϩ�����۵õ�2��3-����-1-��ϩ��B��2��3-����-1-��ϩ��Ϊͬ���칹�壬������̼ԭ�Ӵ���ͬһƽ�森
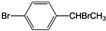
д��B�Ľṹ��ʽ����CH3��2C=C��CH3��2
���һ����2��3-����-1-��ϩ�Ʊ�B�ĺϳ�·�ߣ�CH2=C��CH3��C��CH3��2$��_{һ��������}^{HBr}$��CH3��2CBrCH��CH3��2$��_{��}^{NaOH�Ĵ���Һ}$��CH3��2C=C��CH3��2
���ϳ�·�߳��õı�ʾ��ʽΪ��A$��_{��Ӧ����}^{��Ӧ�Լ�}$B��$��_{��Ӧ����}^{��Ӧ�Լ�}$Ŀ����
���� ��1�����屽��ϩ���ϩ��һ�������·����Ӿ۷�Ӧ���ɸ߷��ӻ����
��2���ұ����� ʱ��������Hԭ�ӱ�ȡ����Ҫ�廯����������֧����Hԭ�ӱ�ȡ����Ҫ����������
ʱ��������Hԭ�ӱ�ȡ����Ҫ�廯����������֧����Hԭ�ӱ�ȡ����Ҫ����������
��3�� ����������������Һ���ȵõ�A��A��������������FeCl3��Һ����ɫ��˵��A��û�з��ǻ�����ֻ��֧����Brԭ�ӱ�-OHȡ�����ݴ��ж�A�ṹ��ʽ��A����������ȥ��Ӧ���ɶ��屽��ϩ��
����������������Һ���ȵõ�A��A��������������FeCl3��Һ����ɫ��˵��A��û�з��ǻ�����ֻ��֧����Brԭ�ӱ�-OHȡ�����ݴ��ж�A�ṹ��ʽ��A����������ȥ��Ӧ���ɶ��屽��ϩ��
��4��B��2��3-����-1-��ϩ��Ϊͬ���칹�壬������̼ԭ�Ӵ���ͬһƽ�棬��B���ĸ�������̼̼˫������̼ԭ�ӣ���ṹ��ʽΪ��CH3��2C=C��CH3��2��
2��3-����-1-��ϩ��HBr�����ӳɷ�Ӧ���ɣ�CH3��2CBrCH��CH3��2����CH3��2CBrCH��CH3��2������ȥ��Ӧ����
��CH3��2C=C��CH3��2��
��� �⣺��1�����屽��ϩ���ϩ��һ�������·����Ӿ۷�Ӧ���ɸ߷��ӻ������ṹ��ʽ ��
�� ��
��
�ʴ�Ϊ��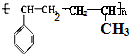 ��
��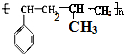 ��
��
��2���ұ����� ʱ��������Hԭ�ӱ�ȡ����Ҫ�廯������������ҪҺ������Ӧ�֧����Hԭ�ӱ�ȡ����Ҫ������������Ҫ������Ӧ��ʴ�Ϊ��Һ�塢�廯�����������塢���գ�
ʱ��������Hԭ�ӱ�ȡ����Ҫ�廯������������ҪҺ������Ӧ�֧����Hԭ�ӱ�ȡ����Ҫ������������Ҫ������Ӧ��ʴ�Ϊ��Һ�塢�廯�����������塢���գ�
��3�� ����������������Һ���ȵõ�A��A��������������FeCl3��Һ����ɫ��˵��A��û�з��ǻ�����ֻ��֧����Brԭ�ӱ�-OHȡ������A�ṹ��ʽΪ
����������������Һ���ȵõ�A��A��������������FeCl3��Һ����ɫ��˵��A��û�з��ǻ�����ֻ��֧����Brԭ�ӱ�-OHȡ������A�ṹ��ʽΪ ��A��Ũ���ᡢ���������·�����ȥ��Ӧ���ɶ��屽��ϩ��
��A��Ũ���ᡢ���������·�����ȥ��Ӧ���ɶ��屽��ϩ��
�ʴ�Ϊ�� ��Ũ���ᡢ���ȣ�
��Ũ���ᡢ���ȣ�
��4��B��2��3-����-1-��ϩ��Ϊͬ���칹�壬������̼ԭ�Ӵ���ͬһƽ�棬��B���ĸ�������̼̼˫������̼ԭ�ӣ���ṹ��ʽΪ��CH3��2C=C��CH3��2��
2��3-����-1-��ϩ��HBr�����ӳɷ�Ӧ���ɣ�CH3��2CBrCH��CH3��2����CH3��2CBrCH��CH3��2������ȥ��Ӧ����
��CH3��2C=C��CH3��2��������ͼΪCH2=C��CH3��C��CH3��2$��_{һ��������}^{HBr}$��CH3��2CBrCH��CH3��2$��_{��}^{NaOH�Ĵ���Һ}$��CH3��2C=C��CH3��2��
�ʴ�Ϊ����CH3��2C=C��CH3��2��CH2=C��CH3��C��CH3��2$��_{һ��������}^{HBr}$��CH3��2CBrCH��CH3��2$��_{��}^{NaOH�Ĵ���Һ}$��CH3��2C=C��CH3��2��
���� ����Ϊ2015��߿��⣬�����л���ѧ�ϳɣ�Ϊ��Ƶ���㣬���ؿ���ѧ��֪ʶ�ۺ�Ӧ����������Ҫѧ���Գ����л�������ż������ʡ�������Ӧ���ͼ������������ղ�������ã��ѵ��Ǻϳ�·����Ƶģ�4���⣬��Ŀ�ѶȽϴ�


��1�����Һ�м���Na2SO3 ��Һ����������ԭ����Ӧ�����ӷ���ʽΪSO32-+I2+H2O=2I-+SO42-+2H+��
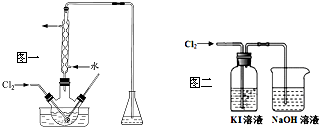
��2����������������������ƿ�н��У���ͼһ��������Һ���������pHԼΪ2������ͨ��Cl2��ʵ����ˮԡ������40�����ҵĽϵ��¶��½��е�ԭ����ʹ��������Һ���нϴ���ܽ�ȣ����ֹI2�������ֹI2��һ������������
��3��ij�о�С����ͼ��װ�ö�Cl2��KI��Һ�ķ�Ӧ����̽��������ͨ��Cl2һ��ʱ���KI��Һ��Ϊ��ɫ������ͨ��Cl2������Һ��ɫ��dz������Ϊ��ɫ���о�С���������ɫ��Һ�е�Ԫ�صĴ�����̬��������¼��裺
����һ��û��I2��̬���������û��I-��̬������������IO3-��̬��
�������ʵ��֤������һ�������Լ���ѡ����
| ʵ����� | Ԥ������ | ���� |
| ����һ���� |
��4�����о�С�黹�����˶Լӵ�����KIO3�����ⶨ������ʵ�飺
��ȷ��ȡ�ӵ���m g���ձ��У�������������ˮ������KI���ٵ���������ϡ���ᣬ��ַ�Ӧ�������û��Һ���250.00mL������Һ����ȡ25.00mL������Һ����ƿ�У��Ӽ��ε�����Һ����c mol•L-1 Na2S2O3��Һ�ζ����յ㣬�ظ�3�Σ����ƽ��ֵΪV mL��
��֪��IO3-+5I-+6H+=3H2O+3I2��I2+2S2O32-=2I-+S4O62-���ⶨʱ���жϴﵽ�ζ��յ������Ϊ��Һ����ɫ��Ϊ��ɫ���Ұ���Ӳ���ɫ���ɲⶨ���ݿ���ø���Ʒ�к�KIO3����������Ϊ$\frac{21.4CV}{6m}$%���ú�m��c��V�Ĵ���ʽ��ʾ��Mr��KIO3��=214 ����
���ڵζ�������ȷ���������£��ô��ֲⶨ������õĽ������ƫ�ߣ���ԭ�����ܿ�����Ӱ�죬�������ӷ���ʽ��ʾ������һӰ���ԭ��4I-+O2+4H+=2I2+2H2O��
| A�� | �ڳ����Ļ������У����̬�ӵ����ߵ�˳���ǣ�C��N��O=S��Cl | |
| B�� | ԭ��������С�����ǣ�C��N��O��S��Cl | |
| C�� | ����Ԫ�����ڱ������������������Ӵ���С���ǣ�Cl��O��S��N��C | |
| D�� | ԭ�ӵ������������ɶ����ٵ�˳���ǣ�Cl��O=S��N��C |
| A�� | þ�� | B�� | �⻯�� | C�� | �� | D�� | ���Ȼ�̼ |
| A�� | Ԫ�ر��ĵ��ʿ�����ұ������ | B�� | ���붡�γɵķ������зǼ��Է��� | ||
| C�� | �����Ӱ뾶�������ң��� | D�� | �������γɵĻ�������������� |
 2H2��g��+O2��g�� ��H=+285.5k•Jmol��1
2H2��g��+O2��g�� ��H=+285.5k•Jmol��1 2NH3��g����H=-38.6k•Jmol��1
2NH3��g����H=-38.6k•Jmol��1