��Ŀ����
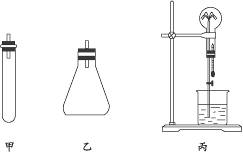
ʵ�����ư���ͨ�������ַ��������ù����������ƺ��Ȼ�立�Ӧ��װ��ͬ�α������ڳ�ȡ7.9 g̼����粒�������Թ��ڣ��ټ���8 g�������ƹ��壬�����¼��ܲ���������װ������ͼ�мף���������ƿ��ע��50 mLŨ��ˮ���ټ���10 g�������ƹ��壨װ������ͼ���ң���ѡȡ����250 mL�ĸ������ƿ��ƿ���������ռ������Ʒ��İ�����������������⣺
��1��д���âڷ���ȡ�����Ļ�ѧ����ʽ��__________________________________________��
��2��˵���â۷���ȡ������ԭ����________________________________________________��
��3�����鰱�����ռ����ķ�����__________________________________________________��
��4���ü�����������ƿ����Ȫʵ�飬װ����ͼ�б����ձ���ʢ��̪��Һ������������_____
_______________________________________________________________________________��

��1��д���âڷ���ȡ�����Ļ�ѧ����ʽ��__________________________________________��
��2��˵���â۷���ȡ������ԭ����________________________________________________��
��3�����鰱�����ռ����ķ�����__________________________________________________��
��4���ü�����������ƿ����Ȫʵ�飬װ����ͼ�б����ձ���ʢ��̪��Һ������������_____
_______________________________________________________________________________��
��1��NH4HCO3+2NaOH====Na2CO3+NH3��+2H2O
(2)NaOH��������ˮ���ȣ��ٽ�NH3��H2O�ֽ�ų�NH3
��3������ʪ��ĺ�ɫʯ����ֽ����ƿ�ڣ���ֽ������֤�����������ò�����պŨ�������ƿ�ڣ��а��̣�֤������NH3
��4���γɺ�ɫ��Ȫ������ƿ����Һ���
(2)NaOH��������ˮ���ȣ��ٽ�NH3��H2O�ֽ�ų�NH3
��3������ʪ��ĺ�ɫʯ����ֽ����ƿ�ڣ���ֽ������֤�����������ò�����պŨ�������ƿ�ڣ��а��̣�֤������NH3
��4���γɺ�ɫ��Ȫ������ƿ����Һ���
ʵ����ȡ����Ļ���Ҫ���ǣ�����ײ�������Ѹ�ٵ���ȡ�ϴ������壬��Ҳ������ֱ�Ӽ���Ũ��ˮ�ķ����Ƶð�����

��ϰ��ϵ�д�
 ����ѧ����ϵ�д�
����ѧ����ϵ�д�
�����Ŀ
 4NO+6H2O,��4NO+3O2+2H2O====4HNO3����ԭ�����������x mol O2���������������y mol HNO3,����������ע��100 mLˮ���õ���Һ��
4NO+6H2O,��4NO+3O2+2H2O====4HNO3����ԭ�����������x mol O2���������������y mol HNO3,����������ע��100 mLˮ���õ���Һ�� ?
?