��Ŀ����
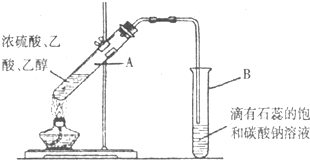
��ʵ�������ǿ�������ͼ����ʾ��װ����ȡ�����������ش��������⣺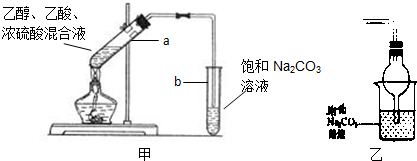
��1�������йظ�ʵ��������У���ȷ����______������ţ�
����a�Թ����ȼ���Ũ���ᣬȻ���ҡ���Թܱ����������Ҵ����ټ�����
�ڱ���̼������Һ���Գ�ȥ���������в���������
������������һ����ɫ��������ζ����״Һ��
����a�Թ��м��뼸�����Ƭ�������Ƿ�ֹ����ʱҺ�屩��
��2��Ũ������˴����������⣬��һ�����ǣ�______
��3��װ����Ͷ�����ĵ���Ҫ���ڱ���̼������Һ��Һ���ϣ����ܲ�����Һ�У���ͬѧ��������ͼ��װ�������վͿɲ�����Һ�У�������Ƿ����______����ǡ�����
��4����Ҫ���Ƶõ������������������Ӧ���õ�ʵ�������______
��5��a�Թ��з�����Ӧ�Ļ�ѧ����ʽ��______��

��1�������йظ�ʵ��������У���ȷ����______������ţ�
����a�Թ����ȼ���Ũ���ᣬȻ���ҡ���Թܱ����������Ҵ����ټ�����
�ڱ���̼������Һ���Գ�ȥ���������в���������
������������һ����ɫ��������ζ����״Һ��
����a�Թ��м��뼸�����Ƭ�������Ƿ�ֹ����ʱҺ�屩��
��2��Ũ������˴����������⣬��һ�����ǣ�______
��3��װ����Ͷ�����ĵ���Ҫ���ڱ���̼������Һ��Һ���ϣ����ܲ�����Һ�У���ͬѧ��������ͼ��װ�������վͿɲ�����Һ�У�������Ƿ����______����ǡ�����
��4����Ҫ���Ƶõ������������������Ӧ���õ�ʵ�������______
��5��a�Թ��з�����Ӧ�Ļ�ѧ����ʽ��______��
��1����Ϊ��ֹ��Һ�ɽ���Ӧ�ȼ����Ҵ���Ȼ���ڼ���Ũ��������ᣬ���ȼ�Ũ����������Һ�ɽ��Ŀ������ʢٴ���
���Ʊ���������ʱ���ñ���̼������Һ��������������Ŀ���dz�ȥ�Ҵ������ᡢ������������������ζ�����������������ܽ�ȣ����ڷֲ㣬�ʢ���ȷ��
�۵ͼ���һ�������ζ����������Ϊ��ɫ��������ζ����״Һ�壬�ʢ���ȷ��
���ڸ����Һ�����ʱ���������Ƭ���Է�ֹ���У��ʢ���ȷ��
��ѡ�ڢۢܣ�
��2����������Ӧ��Ũ�������������⣬������ˮ�������ã���Ϊ�÷�Ӧ�ǿ��淴Ӧ��
�ʴ�Ϊ����ˮ����
��3�����θ���ܵ����ó���ʹ����������������⣬�����Է�ֹ������
�ʴ�Ϊ���ǣ�
��4��������������ʱ�Ƚ�ʢ�л������Թܳ����ʹ���ᡢ�Ҵ��ܽ⣬���÷ֲ��ȡ�ϲ������������
�ʴ�Ϊ����Һ��
��5���������Ҵ���Ũ���������¼��ȷ���������Ӧ��������������ˮ��ͬʱ�÷�Ӧ���棬��Ӧ�Ļ�ѧ����ʽΪ��CH3COOH+C2H5OH
CH3COOC2H5+H2O��
�ʴ�Ϊ��CH3COOH+C2H5OH
CH3COOC2H5+H2O��
���Ʊ���������ʱ���ñ���̼������Һ��������������Ŀ���dz�ȥ�Ҵ������ᡢ������������������ζ�����������������ܽ�ȣ����ڷֲ㣬�ʢ���ȷ��
�۵ͼ���һ�������ζ����������Ϊ��ɫ��������ζ����״Һ�壬�ʢ���ȷ��
���ڸ����Һ�����ʱ���������Ƭ���Է�ֹ���У��ʢ���ȷ��
��ѡ�ڢۢܣ�
��2����������Ӧ��Ũ�������������⣬������ˮ�������ã���Ϊ�÷�Ӧ�ǿ��淴Ӧ��
�ʴ�Ϊ����ˮ����
��3�����θ���ܵ����ó���ʹ����������������⣬�����Է�ֹ������
�ʴ�Ϊ���ǣ�
��4��������������ʱ�Ƚ�ʢ�л������Թܳ����ʹ���ᡢ�Ҵ��ܽ⣬���÷ֲ��ȡ�ϲ������������
�ʴ�Ϊ����Һ��
��5���������Ҵ���Ũ���������¼��ȷ���������Ӧ��������������ˮ��ͬʱ�÷�Ӧ���棬��Ӧ�Ļ�ѧ����ʽΪ��CH3COOH+C2H5OH
| Ũ���� |
| �� |
�ʴ�Ϊ��CH3COOH+C2H5OH
| Ũ���� |
| �� |

��ϰ��ϵ�д�
 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д� ���100�ֵ�Ԫ�Ż�������ϵ�д�
���100�ֵ�Ԫ�Ż�������ϵ�д�
�����Ŀ