��Ŀ����
ͨ������£�����ǻ�����ͬһ��̼ԭ���ϵķ��ӽṹ�Dz��ȶ��ģ������Զ�ʧˮ������̼��˫���Ľṹ��![]()
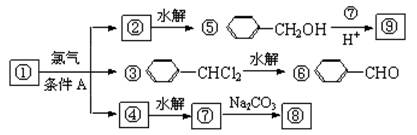
������9���������ת���ϵ��
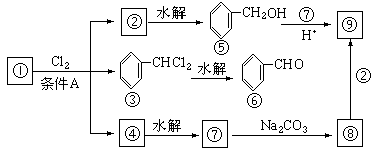
��1�����������__________����������������Ӧ������A��__________��
��2��������ݸ��߿�����Ĵ���ȥˮ���ɻ�����ᣬ ��Ľṹ��ʽ�ǣ�_______��������______________________________________��
��3�������������Ҫ�Ķ���������Ϲ�ҵ�ϳ��û������͢�ֱ�Ӻϳ�����д���˷�Ӧ�Ļ�ѧ����ʽ��
��1���ױ������ա���2��C6H5COOCH2C6H5�������ᱽ������
��3��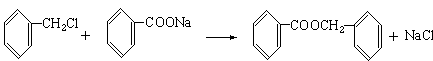
����:
�����е�����Ϣ��ת����ϵͼ��������������һ���µ��龰�����������漰��֪ʶ����������ֻ������������������������Ĵ���ȩ���ᡢ�������ʺ������غ㶨�ɵ�֪ʶ���ݡ�
���ת����ϵͼ�䲻���������������ó����Ǽױ����ڡ��ۡ����Ǽױ��м�����ԭ�ӱ���ȡ����IJ����������֪����C6H5CH2Cl����C6H5CCl3������C6H5COOH�����Ԣ��DZ����ᱽ����������������Ա�̼��ǿ�����Ա�������Ը�̼���Ʒ�Ӧ���ɱ������ƣ���Ӧ���ǣ���������+���״��������ᱽ���������������غ㶨�ɿɵó��÷�Ӧ����һ�ֲ���Ӧ�����Ȼ��ơ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�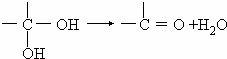
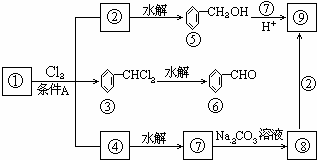
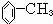
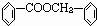
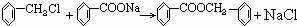
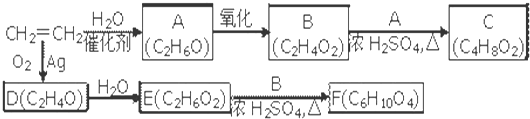
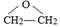
 ������9�ֻ������ת���ϵ��
������9�ֻ������ת���ϵ�� 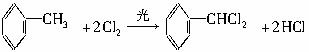 ?
?