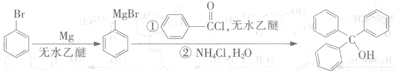
��Ŀ����
��ҵ��������Ĺ������£�
���������գ�
��1������ˮ������������Mg2+��Ca2+�������������A��B������(A��Դ��ʯ��Ҥ�������������B �Ļ�ѧʽΪ ��
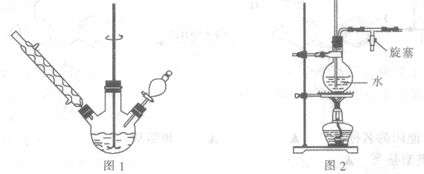
��2��ʵ����ģ������Һ�Ʊ�������װ�����£�
��ͼ1��װ�ú�ͼ2��װ�õ����ӷ���Ϊa�� ��b�� ��f��c��
��ͼ2���Լ�ƿ�ڷ����Ļ�ѧ��Ӧ����ʽΪ ��
��ʵ����Ҫ��ͨ���NH3����֮����ͨ��CO2���壬����ͨ���NH3�ѹ�����ʵ������� ��
��3�����������պ�Ĵ����к���δ�ֽ��̼�����ơ�ijͬѧ��ȡ�ô�����Ʒm g���ٳ�ּ������������ٱ仯ʱ�Ƶ�ʣ����������Ϊn g������Ʒ��̼���Ƶ���������Ϊ ��
��4������25���£�0.1mol/LNH3��H2O��Һ��0.1mol/LNH4Cl��Һ����������Һ�������ϲ����Һ��pH=9������˵����ȷ���� ������ţ���
a��0.1mol/L NH4Cl��Һ���Ϻ���Һ�е������ӵ��������Ŀ����ͬ
b����Ϻ����Һ�У�c(NH3��H2O)��c(Cl-)��c(NH4+)��c(OH-)��c(H+)
c���������֪��NH3��H2O�ĵ���̶ȴ���ͬŨ�ȵ�NH4Cl��ˮ��̶�
d�����ǰ������Һ��pH֮�ʹ���14
��14�֣���1��Na2CO3��2�֣���2����d��2�֣���e��2�֣�
�� NaCl+NH3+CO2+H2O��NaHCO3��+NH4Cl��2�֣�
����պ��Ũ����IJ����������ܿ�f�����а������ɣ�˵����������������ʪ��ĺ�ɫʯ����ֽ����
�ܿ�f������ֽ������˵���������������������𰸾��÷֣���2�֣�
��3��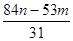 ��100%�����������𰸾��÷֣���2�֣� ��4�� c d��2�֣�
��100%�����������𰸾��÷֣���2�֣� ��4�� c d��2�֣�
���������������1����������Mg2+��Ca2+�ֱ���OH����CO32����ȥ������A��Դ��ʯ��Ҥ������A����ʯ�ҡ����ڲ��������µ����ʣ���BӦ����Na2CO3��
��2����Aװ�����Ʊ�CO2��Bװ�����Ʊ������ġ����ڰ�����������ˮ��ֱ��ͨ����Һ��������������������ȷ����Ӧ����a��d��b��e��f��c��
��ͼ2���Լ�ƿ�����Ʊ�̼�����Ƶģ����Է�����Ӧ�Ļ�ѧ��Ӧ����ʽΪNaCl+NH3+CO2+H2O��NaHCO3��+NH4Cl��
�۰����Ǽ������壬��ʹʪ��ĺ�ɫʯ����ֽ������Ҳ�ܺ��Ȼ��ⷴӦ�����Ȼ�臨�ð���̣����Լ���ͨ���NH3�ѹ�����ʵ���������պ��Ũ����IJ����������ܿ�f�����а������ɣ�˵����������������ʪ��ĺ�ɫʯ����ֽ�����ܿ�f������ֽ������˵������������
��3��̼�����Ʒֽ�ķ���ʽ��
2NaHCO3 Na2CO3��H2O��CO2�� ��m��
Na2CO3��H2O��CO2�� ��m��
2��84g 106g 62g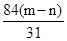 g ��m��n��g
g ��m��n��g
����Ʒ��̼���Ƶ�������mg��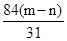 g��
g��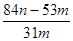 g
g
���Դ�����Ʒ��̼���Ƶ���������Ϊ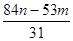 ��100%
��100%
��4��0.1mol/L NH4Cl��Һ���Ϻ���Һ�е������ӵ�������ͬ������Ŀ��ͬ��a����ȷ��25���£�0.1mol/LNH3��H2O��Һ��0.1mol/LNH4Cl��Һ����������Һ�������ϲ����Һ��pH=9����Һ�¼��ԣ���˵����ˮ�ĵ���̶ȴ���NH4����ˮ��̶ȣ���˻�Ϻ����Һ��c(NH4+)��c(Cl-)��c(NH3��H2O)��c(OH-)��c(H+)��b����ȷ��c��ȷ��0.1mol/LNH3?H2O��Һ�Լ��Ժ�0.1mol/LNH4Cl��Һ�����ԣ�NH4����ˮ��̶�С�ڰ�ˮ�ĵ���̶ȣ����ǰ������Һ��pH֮�ʹ���14����d��ȷ����ѡcd��
���㣺������ε��ᴿ��̼�����Ƶ��Ʊ��������ļ��顢���ʺ����ļ��㡢��Һ������Ũ�ȵĴ�С�Ƚϵ�

 ����С״Ԫ��������������ϵ�д�
����С״Ԫ��������������ϵ�д�NaCl��NaClO�����������¿ɷ�����Ӧ��ClO��+Cl��+2H+ = Cl2��+H2O��ijѧϰС�����о�����Һ(��Ҫ�ɷ�ΪNaCl��NaClO)�ı��������
��1��������Һ��NaClO�����տ����е�CO2����NaHCO3��HClO�����ʡ�д����ѧ��Ӧ����ʽ ��
��2��ȡ��������Һ�����Թ��У���������һ��Ũ�ȵ����ᣬ������ų���ͨ������װ�ü�������ijɷֿ����ж�����Һ�Ƿ���ʡ�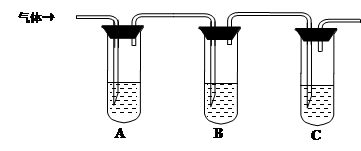
��ѡ�Լ���98%Ũ���ᡢ1%Ʒ����Һ��1.0 mol��L��1 KI-������Һ��1.0 mol��L��1NaOH������ʯ��ˮ������NaCl��Һ
���������ʵ�鷽����
| �����Լ� | Ԥ������ͽ��� |
| �Թ�A�м������� �� �Թ�B�м�1%Ʒ����Һ�� �Թ�C�мӢ� �� | ��A����Һ����ɫ��B����Һ����ɫ��C����Һ����ǡ�������Һ���ֱ��ʣ� �� ������Һδ���ʣ� �� ������Һ��ȫ���ʡ� |
��3���õζ����ⶨ����Һ��NaClO��Ũ�ȡ�ʵ�鲽�����£�
����ȡ 25.00mL����Һ������ƿ�У����������a mol��L��1 Na2SO3��Һb mL��
�ڵζ���������c mol��L��1������KMnO4��Һװ�� ������ʽ���ʽ���ζ����У�KMnO4��ʣ���Na2SO3������Ӧ������Һ����ɫ���dz��ɫ���ұ��ְ�����ں�ɫ����ʱ��ֹͣ�ζ�����¼���ݡ��ظ��ζ�ʵ��2�Σ�ƽ����������KMnO4��Һv mL��
�ζ��������漰�ķ�Ӧ�У�NaClO + Na2SO3 = NaCl+ Na2SO4 ��
2KMnO4 + 5Na2SO3+ 3H2SO4 = K2SO4 + 2MnSO4 + 5Na2SO4 + 3H2O
�ۼ��㡣����Һ��NaClO��Ũ��Ϊ mol��L��1���ú�a��b��c��v�Ĵ���ʽ��ʾ����
ijͭ��ʯ��ͭԪ�غ����ϵͣ��Һ�������þ���Ƶ��������ӡ�ijС����ʵ�������ý���-��ȡ���Ʊ�����ͭ��
��1������IΪ_______������II�õ��IJ����������ձ�_______
��2������II������III����ҪĿ����_______������ͭԪ�ء�
��3��С���Ա����CuSO4��Һ��Na2CO3��Һ��Ϸ�Ӧ���Ʊ�������ľ�ķ�����Cu2(OH)2CO3����Һ�����ʵ�鷢��������ɫ����Һ��ɫ���в��죬�������ϱ��������������������Ʋ�ͬʹ���л��н϶�Cu(OH)2��Cu4(OH)6SO4��
��֪Cu(OH)2��Cu2(OH)2CO3��Cu4((OH)6SO4��������ˮ����������ֽ��¶�����Ϊ 80�桢200�桢300�档
���ʵ���������Һ�ɷ֣���ɱ������ݡ�
��ѡ�Լ���2mol?L��1HCl��1 mol?L��1H2SO4��0.1 mol?L��1NaOH��0.1 mol?L��1 BaCl2������ˮ����������Ʒ��ѡ��
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡ��������Һ�����ˣ����ϴ�Ӻ�ȡ�������Թ��У�_______________________________________________________________ | ˵������Һ�л�__________________________________________,��Cu4( OH)6SO4�� |
| ����2����ȡ��������Һ���Թ��У�____________________________ | ______________�� ˵������Һ�л���Cu( OH) 2�� |
��4������ʵ����Ҫ100mL 0.5 mol?L��1��CuSO4��Һ������ʱ���ȡ_______gCuSO4?5H2O (��ѧʽ����250)��
�������ȣ�ClO2����Ŀǰ�����Ϲ��ϵĵ��Ĵ���Ч�����Ĺ�������������һ�ֻ���ɫ�����壬������ˮ��ʵ���ҿ���NH4Cl�����ᡢNaClO2���������ƣ�Ϊԭ�����Ʊ�ClO2�����������£�
��1��д�����ʱ������Ӧ�Ļ�ѧ����ʽ�� ��
��2����ȥClO2�е�NH3��ѡ�õ��Լ��� ��������ĸ��
| A������ʳ��ˮ | B����ʯ�� | C��Ũ���� | D��ˮ |
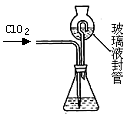
��װ���в���Һ��ܵ������� �� ��
����д��������������������⻯����Һ��Ӧ�����ӷ���ʽ �� ��
�۵ζ��յ�������ǣ� ��
�ܲ��ͨ��ClO2������m(ClO2)= �����ú�c��V�Ĵ���ʽ��ʾ������֪��ClO2����Է�������Ϊ67.5��
��4�����ʵ����ȷ����ҺX�ijɷ֣��벹�����ʵ�鲽�������
| ʵ�鲽�� | ʵ������ | ʵ����� |
| �� | | ��ҺX�к���Na+ |
| �� | | ��ҺX�к���Cl�� |
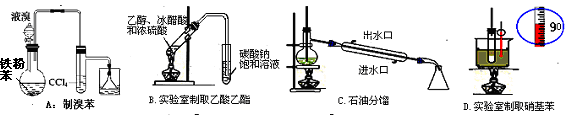
�����й�ʵ��װ�ý��е���Ӧʵ�飬�ܴﵽʵ��Ŀ�ĵ���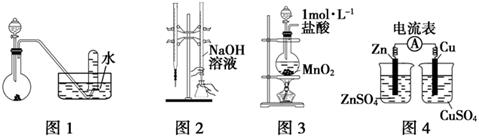
| A����ͼ1��ʾװ�ý���ϡ������ͭ�ķ�Ӧ��ȡ���ռ�NO |
| B����ͼ2��ʾװ�ý�������֪Ũ�ȵ�����������Һ�ⶨ����Ũ�ȵ�ʵ�� |
| C����ͼ3��ʾװ����ȡ����Cl2 |
| D����ͼ4��ʾװ�ü�������ķ��� |
���������У��ȿ���Ũ�������ֿ��ù����������Ƹ������
| A��Cl2 | B��SO2 | C��H2 | D��NH3 |