��Ŀ����
�����£��� 1L 0.10 mol��L-1CH3COONa ��Һ�У�����ͨ��HC1���壨������Һ����仯�����õ� c (CH3COO-)��c(CH3COOH)�� pH �ı仯��ϵ���£�������˵����ȷ����

A. ��Һ��pH�Ƚϣ�x<y <z
B. ��y����ͨ��0.05 mol HCl���壬��Һ������Ũ�ȴ�С�Ƚϣ�c(Na+) =c(Cl-) > c(H+) >c(CH3COO-) >c(OH-)
C. ����Һ�м���һ��ǿ���ǿ���ҺpH�仯��С����y��
D. ���¶��£�CH3COOH��Ka=104.75
��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
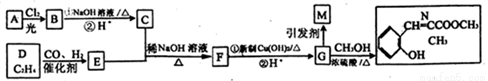
�����Ŀ



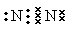
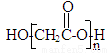
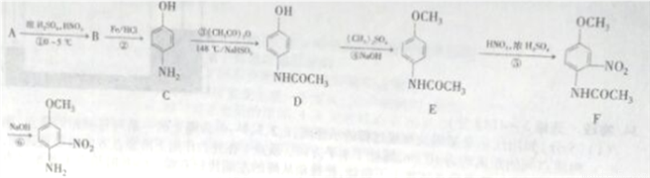
 NH2���������ԣ��ױ�������
NH2���������ԣ��ױ������� �ṹ���ܷ���������Ӧ
�ṹ���ܷ���������Ӧ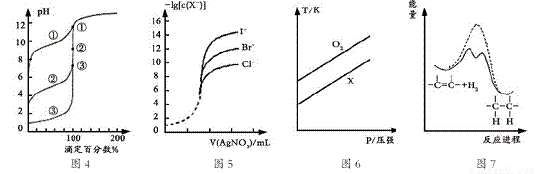
 R1CH=
R1CH=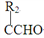 +H2O
+H2O