��Ŀ����
����Ŀ������ϩ���������Ϻͺϳ�����Ҫ�����л�ԭ�ϣ��ұ������ⷨ��Ŀǰ��������������ϩ����Ҫ�������仯ѧ����ʽΪ��
![]()
��1���������¶ȣ��÷�Ӧ��ƽ�ⳣ�������H_______0������ڡ���С�ڡ������÷�Ӧ��_______________���������Է����С�
��2��ά����ϵ��ѹǿ�Ѻ㶨�����¶�Tʱ�����ʵ���Ϊ2mol�����Ϊ1L���ұ��������������ⷴӦ����֪�ұ���ƽ��ת����Ϊ80%�����ڸ��¶��·�Ӧ��ƽ�ⳣ��K=_____��
��3�������Ϊ2L�ĺ����ܱ�������ͨ��2mol�ұ�������2���Ӻ�ﵽƽ�⣬���������Ũ����0.5mol/L�����ұ������ķ�Ӧ����Ϊ_________________��ά���¶Ⱥ�����������䣬������ƽ������ͨ��1mol������1mol�ұ���������v��_______v�棨����ڡ�����С�ڡ����ڡ���
���𰸡� ���� �ϸ��¶� 32/9 0.25mol��L-1��min-1 ����
����������1�������¶ȣ��÷�Ӧ��ƽ�ⳣ�����������Ӧ���ȣ���H��0����Ӧ�Է���Ҫ���ǡ�G=��H��T��s��0���÷�Ӧ����������ʵ������ӵķ�Ӧ�����ԡ�s��0����֪��H��0�������¶�Խ��Խ�����ڡ�G=��H��T��s��0�����Է�Ӧ����Ϊ�ϸ��¶ȡ�
��2����ʼ��2mol�ұ���ƽ��ת����Ϊ80%����Ӧ���ұ�Ϊ1.6mol�����ɱ���ϩ��������1.6mol��ʣ��0.4mol�ұ�������ƽ��ʱ��һ����1.6+1.6+0.4=3.6mol���塣��ѹ�����������ȵ������ʵ����ıȣ���ʼ2mol�ұ������Ϊ1L������ƽ���3.6mol��������Ϊ1.8L������ƽ�ⳣ��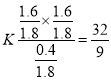 ��
��
��3��![]() mol��L-1��min-1��ԭƽ��̬���ұ��ij�ʼŨ��Ӧ��Ϊ1mol/L��ƽ��ʱ����Ũ��Ϊ0.5mol/L�����Ա���ϩ��Ũ��Ҳ��0.5mol/L��ʣ���ұ���Ũ��Ϊ0.5mol/L��ƽ�ⳣ��K=0.5��0.5/0.5=0.5����ͨ��1mol������1mol�ұ��������ұ�������ϩ�����������ʵ���Ũ��ת��Ϊ1mol/L��0.5mol/L��1mol/L����Q=0.5��1/1=0.5=K������ƽ�ⲻ�ƶ���
mol��L-1��min-1��ԭƽ��̬���ұ��ij�ʼŨ��Ӧ��Ϊ1mol/L��ƽ��ʱ����Ũ��Ϊ0.5mol/L�����Ա���ϩ��Ũ��Ҳ��0.5mol/L��ʣ���ұ���Ũ��Ϊ0.5mol/L��ƽ�ⳣ��K=0.5��0.5/0.5=0.5����ͨ��1mol������1mol�ұ��������ұ�������ϩ�����������ʵ���Ũ��ת��Ϊ1mol/L��0.5mol/L��1mol/L����Q=0.5��1/1=0.5=K������ƽ�ⲻ�ƶ���



