��Ŀ����
����Һ�������25�棬�й�������ȷ����
| A��ij���ʵ���ҺpH<7���������һ�������ǿ�������� |
| B��pH=4��5�ķ���֭��c(H������pH=6��5��ţ����c��H��)��102�� |
| C������ʱ��ij��Һ����ˮ���������c(H��)��c(OH��)�ij˻�Ϊ1��10��24������Һ��һ�����Դ�������K����Na����AlO2����SO42�� |
| D������ʱ��0��l mol/L HA��Һ��pH>l��0��1 mol/L BOH��Һ��c(OH��)/c(H��)=l012������������Һ�������ϣ���Ϻ���Һ������Ũ�ȵĴ�С��ϵΪ��c��B����>c��OH����>c��H����>c��A���� |
B
���������ij���ʵ���ҺpH<7��������ʳ��������ǿ�������Σ����п�������ʽ�Σ�Aѡ�����pH=4.5�ķ���֭��c(H+)��10��4.5��pH=6.5��ţ����c(H+)��10��6.5��10��4.5��10��6.5��100��Bѡ����ȷ������ʱ��ij��Һ����ˮ���������c(H��)��c(OH��)�ij˻�Ϊ1��10��24����c(H��)��c(OH��)��1��10��12����������������Һ��Ҳ�����Ǽ�����Һ��AlO2�����ڼ�����Һ���ܴ���������Һ������Ӧ��˵����Һ�п��ܴ�������K����Na����AlO2����SO42��������˵һ����Cѡ�����0.1mol��L��1 HA��Һ��pH��1��˵��HA�����ᣬ0.1mol��L��1 BOH��Һ��c��OH����/c��H����=1012����������ˮ�����ӻ����������c��OH������0.1mol��L��1�����Լ���ǿ���Ӧ����Һ���ɵ���ABˮ���Լ��ԣ�����ˮ��̶�һ�㶼�Ƚ�С����������Ũ�ȵĴ�С˳��Ϊc��B������c��A������c��OH������c��H������Dѡ�����

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
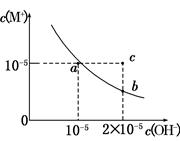
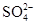
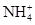 ��Ba2+��
��Ba2+��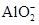 ��Cl-
��Cl-