��Ŀ����
14��A��B��C��D�����ֶ�����Ԫ�أ����ǵ�ԭ�����������������У�A��C��B��D�ֱ���ͬ����Ԫ�أ�AԪ�ص�ԭ�Ӱ뾶����������Ԫ����ԭ�Ӱ뾶��С�ģ�B��D��Ԫ�ص�ԭ�Ӻ���������֮����A��C��Ԫ��ԭ�Ӻ���������֮�͵�2��������Ԫ�����γɵĵ�����A��B���������壬C��D�����ǹ��壮��1��д������Ԫ�ص����ƣ�B����C�ƣ�
��2����B��C��Ԫ�����γɵ�ԭ�Ӹ�����Ϊ1��1�Ļ������������ӣ�����ӡ����ۡ�����������ڵĻ�ѧ�������������Ӽ����Ǽ��Լ������й��ۼ���Ҫд������Ĺ��ۼ����ͣ���д������ˮ��Ӧ�����ӷ���ʽ2Na2O2+2H2O=4Na++4OH-+O2����
��3���õ���ʽ��ʾC2D���γɹ��̣�
 ��
����4��ʵ���ҳ���A��B��Ԫ�����γɵ�ԭ�Ӹ���Ϊ1��1�Ļ��������Ʊ�һ�ֳ������壬д��ʵ�������ù���ҩƷ�Ʊ�������Ļ�ѧ����ʽ2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2����
��5��д��B��ԭ�ӽṹʾ��ͼ
 ��һ��Bԭ������8�����ӣ�д��B��ԭ�ӷ���168O��
��һ��Bԭ������8�����ӣ�д��B��ԭ�ӷ���168O��
���� A��B��C��D�����ֶ�����Ԫ�أ����ǵ�ԭ��������������AԪ�ص�ԭ�Ӱ뾶����������Ԫ����ԭ�Ӱ뾶��С����AΪ��Ԫ�أ�A��C��B��D�ֱ���ͬ����Ԫ�أ�B��D��Ԫ�ص�ԭ�Ӻ���������֮����A��C��Ԫ��ԭ�Ӻ���������֮�͵�2������B��D���ڶ��������ڣ���C���ڵ������ڣ���CΪ��Ԫ�أ�B��D�ֱ���ͬ����Ԫ�أ�B��D��Ԫ�ص�ԭ�Ӻ���������֮��Ϊ2��1+11��=24����BΪ��Ԫ�أ�DΪ��Ԫ�أ���������Ԫ�����γɵĵ�����A��B���������壬C��D�����ǹ��壬�������⣮
��� �⣺A��B��C��D�����ֶ�����Ԫ�أ����ǵ�ԭ��������������AԪ�ص�ԭ�Ӱ뾶����������Ԫ����ԭ�Ӱ뾶��С����AΪ��Ԫ�أ�A��C��B��D�ֱ���ͬ����Ԫ�أ�B��D��Ԫ�ص�ԭ�Ӻ���������֮����A��C��Ԫ��ԭ�Ӻ���������֮�͵�2������B��D���ڶ��������ڣ���C���ڵ������ڣ���CΪ��Ԫ�أ�B��D�ֱ���ͬ����Ԫ�أ�B��D��Ԫ�ص�ԭ�Ӻ���������֮��Ϊ2��1+11��=24����BΪ��Ԫ�أ�DΪ��Ԫ�أ���������Ԫ�����γɵĵ�����A��B���������壬C��D�����ǹ��壬�������⣮
��AΪ��Ԫ�أ�BΪ��Ԫ�أ�CΪ��Ԫ�أ�DΪ��Ԫ�أ�
��1��������������֪��BΪ��Ԫ�أ�CΪ��Ԫ�أ��ʴ�Ϊ�������ƣ�
��2��BΪ��Ԫ�أ�CΪ��Ԫ�أ�����Ԫ�����γɵ�ԭ�Ӹ�����Ϊ1��1�Ļ�����ΪNa2O2��������������������ӹ��ɣ��������ӻ���������������������֮��Ϊ���Ӽ�����������������ԭ��֮��Ϊ�Ǽ��Լ�������������ˮ��Ӧ����������������������Ӧ���ӷ���ʽΪ2Na2O2+2H2O=4Na++4OH-+O2����
�ʴ�Ϊ�����ӣ����Ӽ����Ǽ��Լ���2Na2O2+2H2O=4Na++4OH-+O2����
��3��C2DΪNa2S�����������������ӹ��ɣ�����ʽ��ʾNa2S���γɹ���Ϊ ��
��
�ʴ�Ϊ�� ��
��
��4��AΪ��Ԫ�أ�BΪ��Ԫ�أ���Ԫ�����γɵ�ԭ�Ӹ���Ϊ1��1�Ļ�����ΪH2O2��ʵ���ҳ���H2O2����O2����Ӧ����ʽΪ2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2����
�ʴ�Ϊ��2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2����
��5��BΪ��Ԫ�أ�B��ԭ�ӽṹʾ��ͼΪ ����һ��Bԭ������8�����ӣ���B��ԭ�ӷ���Ϊ168O���ʴ�Ϊ��
����һ��Bԭ������8�����ӣ���B��ԭ�ӷ���Ϊ168O���ʴ�Ϊ�� ��168O��
��168O��
���� ������Ԫ���ƶ�Ϊ���壬����ѧ������ԭ�ӵĽṹ���ƶ�Ԫ�ء����û�ѧ����ȣ�ע�Ȿ��������A��C��B��D��������֮��ϵ�����ǽ������ѵ㣬�Ѷ��еȣ�

 ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д�| �¶� | X��Һ�������mL�� | X��Һ��Ũ�ȣ�mol/L�� | ˮ�������mL�� | |
| A | 10�� | 20 | 3 | 10 |
| B | 20�� | 30 | 2 | 0 |
| C | 20�� | 10 | 4 | 20 |
| D | 10�� | 10 | 2 | 20 |
| A�� | A | B�� | B | C�� | C | D�� | D |
| ���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ����A | 0 |
| 2 | E | R | F | |||||
| 3 | A | C | D | H | I | G | ||
| 4 | B |
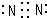 ��A������������Ӧ��ˮ����ĵ���ʽ
��A������������Ӧ��ˮ����ĵ���ʽ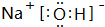 ��
����2��A��C��D����Ԫ�ص��������Ӧ��ˮ�����м�����ǿ����NaOH ���ѧʽ����H��I��G����Ԫ���⻯���У����ȶ�����HCl���ѧʽ����
��3��A��B��C����Ԫ�ص������Ӱ����Ӱ뾶�ɴ�С��˳������ΪK+��Na+��Mg2+������Ԫ�ص����ӷ��ű�ʾ����
��4��д��A�ĵ�����ˮ��Ӧ�Ļ�ѧ����ʽ2Na+2H2O=2NaOH+H2����
��5��XԪ����A��R����Ԫ���е�һ�֣�X��ԭ�Ӻ�����14�����ӣ�2.7gX��������ȼ��ʱ����������2.4g��X���������������������������Һ�з�Ӧ��Ҳ�������ᷴӦ��X��ԭ�ӽṹʾ��ͼ��
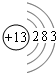 ��X�ĵ��ʺ�����������Һ��Ӧ�����ӷ���ʽΪ2Al+2OH-+2H2O=2AlO2-+3H2����
��X�ĵ��ʺ�����������Һ��Ӧ�����ӷ���ʽΪ2Al+2OH-+2H2O=2AlO2-+3H2���� | A�� | ȩ���ĵ���ʽ��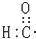 | B�� | ��ϩ�Ľṹ��ʽ��CH2CH2 | ||
| C�� | 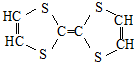 �����л��� �����л��� | D�� | �۱�ϩ�Ļ�ѧʽ��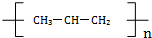 |
| A�� | ���ʯ��ʯī | B�� | H��D��T | C�� | 1940K��2040Ca | D�� | O2��O3 |
| A�� | 2 | B�� | 3 | C�� | 4 | D�� | 5 |

 ���ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�ã���ͼ��ʾһ�����أ�װ�е��Һa��a�DZ���NaCl��Һ��ʵ�鿪ʼʱ��ͬʱ�����߸����뼸�η�̪��Һ��X��Y���Ƕ��Ե缫��ͨ��������ֱ����Դ��������ش��������⣺
���ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�ã���ͼ��ʾһ�����أ�װ�е��Һa��a�DZ���NaCl��Һ��ʵ�鿪ʼʱ��ͬʱ�����߸����뼸�η�̪��Һ��X��Y���Ƕ��Ե缫��ͨ��������ֱ����Դ��������ش��������⣺