��Ŀ����
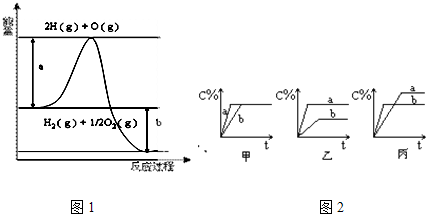
�����Ȼ�ѧ����ʽ����101kPaʱ��S��s��+O2��g��=SO2��g����H=-297.23kJ/mol����������˵���в���ȷ���ǣ�������
| A��S��ȼ����Ϊ297.23��kJ/mol |
| B��S��g��+O2��g��=SO2��g��1molS��g���ų�����������297.23kJ |
| C��S��g��+O2��g��=SO2��g��1molS��g���ų�������С��297.23kJ |
| D���γ�1molSO2�Ļ�ѧ���ͷŵ����������ڶ���1molS��s����1molO2��g���Ļ�ѧ�������յ������� |
��S��s��+O2��g���TSO2��g����H=-297.23kJ/mol��
A����������Ϊ�ȶ������S��ȼ����Ϊ297.23 kJ/mol����A��ȷ��
B���ɸ�˹���ɿ�֪��S��g��+O2��g���TSO2��g����H��-297.23kJ/mol����ų�����������297.23 kJ����B��ȷ��
C���ɸ�˹���ɿ�֪��S��g��+O2��g���TSO2��g����H��-297.23kJ/mol����ų�����������297.23 kJ����C����
D���÷�ӦΪ���ȷ�Ӧ�����γ�1 mol SO2�Ļ�ѧ�����ͷŵ����������ڶ���1 mol S��s����1 mol O2��g���Ļ�ѧ�������յ�����������D��ȷ��
��ѡC��
A����������Ϊ�ȶ������S��ȼ����Ϊ297.23 kJ/mol����A��ȷ��
B���ɸ�˹���ɿ�֪��S��g��+O2��g���TSO2��g����H��-297.23kJ/mol����ų�����������297.23 kJ����B��ȷ��
C���ɸ�˹���ɿ�֪��S��g��+O2��g���TSO2��g����H��-297.23kJ/mol����ų�����������297.23 kJ����C����
D���÷�ӦΪ���ȷ�Ӧ�����γ�1 mol SO2�Ļ�ѧ�����ͷŵ����������ڶ���1 mol S��s����1 mol O2��g���Ļ�ѧ�������յ�����������D��ȷ��
��ѡC��

��ϰ��ϵ�д�
�����Ŀ