��Ŀ����
 ����A��B��C���ֶ�����Ԫ�أ�ԭ���������ε�����A��C��������֮��Ϊ27������������֮��Ϊ5��0.9g����B���������ᷴӦ���ռ�������1.12L����״��������ش��������⣺
����A��B��C���ֶ�����Ԫ�أ�ԭ���������ε�����A��C��������֮��Ϊ27������������֮��Ϊ5��0.9g����B���������ᷴӦ���ռ�������1.12L����״��������ش��������⣺��1��BԪ�صķ���
Al
Al
������2��A��B��C����Ԫ������������Ӧ��ˮ�������Խ�ǿ�����Խ�����˳���ǣ�д��ѧʽ��
NaOH��Al��OH��3��H2SO4
NaOH��Al��OH��3��H2SO4
����3��A��B��Ԫ�ص�����������Ӧ��ˮ����֮����Է�����Ӧ�������ӷ���ʽ
Al��OH��3+OH-=AlO2-+2H2O
Al��OH��3+OH-=AlO2-+2H2O
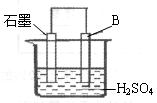
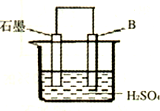
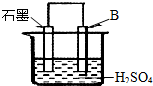
����4����B������ʯī������ͼװ�����ӣ�ʯī�缫������Ϊ
����
����
���õ缫��ӦʽΪ��2H++2e-=H2��
2H++2e-=H2��
����������B�����ԭ������ΪMr�����ϼ�Ϊx�����ݵ���ת�Ƶã�
��x=
��2����Mr=9x��BΪ�����ڽ�����x=1��Mr=9�����������⣻x=2��Mr=18�����������⣻x=3��Mr=27���������⣬��BΪAlԪ�أ���A��C����ͬ���ڣ�C��ԭ����������13����C���ڵ������ڡ�A���ڵڶ����ڣ�û��Ԫ��ͬʱ����������֮��Ϊ27������������֮��Ϊ5����A��C��ͬ���ڣ���C+A=27��C-A=5���ɵ�C=16��A=11����AΪ�ơ�BΪ�ݴ˽��
| 0.9 |
| Mr |
| 1.12 |
| 22.4 |
����⣺��B�����ԭ������ΪMr�����ϼ�Ϊx�����ݵ���ת�Ƶã�
��x=
��2����Mr=9x��BΪ�����ڽ�����x=1��Mr=9�����������⣻x=2��Mr=18�����������⣻x=3��Mr=27���������⣬��BΪAlԪ�أ���A��C����ͬ���ڣ�C��ԭ����������13����C���ڵ������ڡ�A���ڵڶ����ڣ�û��Ԫ��ͬʱ����������֮��Ϊ27������������֮��Ϊ5����A��C��ͬ���ڣ���C+A=27��C-A=5���ɵ�C=16��A=11����AΪ�ơ�BΪ��
��1��������������֪��BΪAl���ʴ�Ϊ��Al��
��2��NaOH����ǿ�Al��OH��3���������������H2SO4 ��ǿ�ᣬ�����Խ�ǿ�����Խ�����˳��NaOH��Al��OH��3��H2SO4 ��
�ʴ�Ϊ��NaOH��Al��OH��3��H2SO4 ��
��3���������ƿ�������������Ӧ�����ӷ���ʽΪ��Al��OH��3+OH-=AlO2-+2H2O��
�ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2H2O��
��4��ԭ��ر�����Al�����ᷴӦ������������������Al����������Ӧ������������ʯīΪ�������������������ŵ������������缫��Ӧʽ��2H++2e-=H2����
�ʴ�Ϊ��������2H++2e-=H2����
| 0.9 |
| Mr |
| 1.12 |
| 22.4 |
��1��������������֪��BΪAl���ʴ�Ϊ��Al��
��2��NaOH����ǿ�Al��OH��3���������������H2SO4 ��ǿ�ᣬ�����Խ�ǿ�����Խ�����˳��NaOH��Al��OH��3��H2SO4 ��
�ʴ�Ϊ��NaOH��Al��OH��3��H2SO4 ��
��3���������ƿ�������������Ӧ�����ӷ���ʽΪ��Al��OH��3+OH-=AlO2-+2H2O��
�ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2H2O��
��4��ԭ��ر�����Al�����ᷴӦ������������������Al����������Ӧ������������ʯīΪ�������������������ŵ������������缫��Ӧʽ��2H++2e-=H2����
�ʴ�Ϊ��������2H++2e-=H2����
���������⿼��ԭ�ӽṹ��Ԫ�����ڱ��Ĺ�ϵ���ƶ�Ԫ���ǽ���Ĺؼ���ע���������Ԫ�����ڱ��Ľṹ���Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
 ����A��B��C���ֶ�����Ԫ�أ�ԭ���������ε�����A��C��������֮��Ϊ27������������֮��Ϊ5��0.9g����B���������ᷴӦ���ռ�������1.12L����״��������ش��������⣺
����A��B��C���ֶ�����Ԫ�أ�ԭ���������ε�����A��C��������֮��Ϊ27������������֮��Ϊ5��0.9g����B���������ᷴӦ���ռ�������1.12L����״��������ش��������⣺