��Ŀ����
(1)�����й�ʵ��������жϲ���ȷ����________(���й���ţ�ѡ�����۷�)��
A����10mL��Ͳ��ȡϡ������Һ8.0mL
B���ø����pH��ֽ�ⶨ��ˮ��pH
C���ü�ʽ�ζ�����ȡKMnO4��Һ19.60mL
D��ʹ������ƿ������Һʱ������Һ�涨�ݺ�������Һ��Ũ��ƫ��
E��Բ����ƿ����ƿ�����������ʱ��Ӧ����ʯ������
F���ⶨ����ͭ�����нᾧˮ����ʱ�������Ⱥ���������ڸ���������ȴ���ٳ���
G���ñ����ᣬ����ˮ������ƿ����������pHΪ1�Ĵ���ϡ��Һ
(2)ʵ������������ͼװ�ý����к��ȵIJⶨ��

�ش��������⣺
�ٸ�ͼ��������δ������������________��________��
�������0.5mol/L��������������ƹ������ʵ�飬��ʵ����������ġ��к��ȡ���(�ƫ����ƫС�����䡱)��ԭ����________��
(3)�ڷ�����ѧ�г���Na2C2O4������Ϊ�����ʲⶨKMnO4��Һ��Ũ�ȣ���H2SO4��Һ�У���Ӧ���£�
2MnO4����5C2O42����16H+![]() 2Mn2+��10CO2����8H2O
2Mn2+��10CO2����8H2O
����������ƽ��ȡWgNa2C2O4���壮
�ڽ�WgNa2C2O4���100mL����Һ����ȡ20.00mL������ƿ�У�������KMnO4��ҺӦװ��________(���ʽ����ʽ��)�ζ����У�
�����ζ��ܵ���ʼ�������յ������ͼ��ʾ��������KMnO4�����ʵ���Ũ��Ϊ________(�����ʽ)��
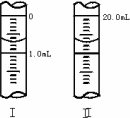
�����ζ���������������ⶨ��KMnO4��Һ��Ũ��________(�ƫ�ߡ���ƫ�͡����䡱)��
������
(1)BCEG
(2)�ٻ��β�����,�ձ��Ϸ�����ĭ���ϸǻ�Ӳֽ���
��ƫ��,����NaOH����ˮ����
(3)����ʽ,��2W/67mol��L��1?,��ƫ��

 ����ͼ��ʾΪ���������IJ��ֽṹ��
����ͼ��ʾΪ���������IJ��ֽṹ��


