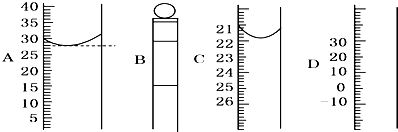
��Ŀ����
 ����ͼ��ʾΪ���������IJ��ֽṹ��
����ͼ��ʾΪ���������IJ��ֽṹ����1����д���������������ƣ�A
��Ͳ
��Ͳ
��B����ƿ
����ƿ
��C�¶ȼ�
�¶ȼ�
����2������B�ϱ����
�ڢۢ�
�ڢۢ�
������ţ��������� ���¶� �ۿ̶��� ��Ũ�� ���ݻ�
��3������Bʹ��ǰ����
����Ƿ�©ˮ
����Ƿ�©ˮ
��������98%��ŨH2SO4���ܶ�Ϊ1.84g/cm3��������480mL0.2mol/L��ϡH2SO4��
�йز���Ϊ���ټ�������Ũ�������� ����ȡһ�������Ũ���� ��ϡ�͡���ȴ ��ת�ơ�ϴ�� �ݶ��� ��ҡ��
�ش���������
��4��Ӧ��ȡ��Ũ���������
5.4mL
5.4mL
��ʵ�������õIJ����������ձ�������������Ͳ����ͷ�ι����500mL����ƿ
500mL����ƿ
����5���ڢ۲���ϡ��Ũ����IJ�����
��Ũ�������ձ��ڱ���������ˮ�У��ӱ��ò���������
��Ũ�������ձ��ڱ���������ˮ�У��ӱ��ò���������
��6���������Ƶ�ϡH2SO4���вⶨ������ʵ��Ũ�ȴ���0.2mol/L���������������Щ��������������Ũ��ƫ����д��ĸ��
ACF
ACF
��A������Ͳ��ȡŨ����ʱ��������Ͳ�Ŀ̶�
B������ƿδ���T����������Һ
C��Ũ�������ձ���ϡ�ͺ�δ��ȴ������ת�Ƶ�����ƿ�У������ж���
D��������ƿת��ʱ��������Һ�彦��
E���ձ�δ����ϴ��
F��������ƿ�ж���ʱ��������ƿ�̶���
G�����ݺ�����ƿ������ҡ�ȣ����ú�Һ�治���̶��ߣ��ټ�ˮ���̶��ߣ�
��������������ƿ�Ľṹ����ʹ��ע���������ƿ�ϱ�����¶ȡ��̶��ߡ��ݻ�������������Һ���ѡ����ʵ�����ƿ������ƿ��ʹ��ǰҪ����Ƿ�©ˮ�ȣ�
����98%��ŨH2SO4���ܶ�Ϊ1.84g/cm3��������480mL0.2mol/L��ϡH2SO4��
��4���������Ʋ����������Һ�����ѡ�����ò�������Ϊ�ձ�������������Ͳ����ͷ�ιܺ�500mL����ƿ������ϡ�Ͷ��ɣ�ϡ��ǰ�����ʵ����������Ӧ��ȡŨ����������
��5��Ũ�����ܶȱ�ˮ������ˮ�ų��������ȣ�ϡ��ʱ��Ũ�������ձ��ڱ���������ˮ�У��ӱ��ò��������裻
��6������c�����ᣩ=
�������ʵ������ӻ���Һ�������Сʱ��ʵ���ܶ�ƫ���������жϣ�
����98%��ŨH2SO4���ܶ�Ϊ1.84g/cm3��������480mL0.2mol/L��ϡH2SO4��
��4���������Ʋ����������Һ�����ѡ�����ò�������Ϊ�ձ�������������Ͳ����ͷ�ιܺ�500mL����ƿ������ϡ�Ͷ��ɣ�ϡ��ǰ�����ʵ����������Ӧ��ȡŨ����������
��5��Ũ�����ܶȱ�ˮ������ˮ�ų��������ȣ�ϡ��ʱ��Ũ�������ձ��ڱ���������ˮ�У��ӱ��ò��������裻
��6������c�����ᣩ=
| n(����) |
| V |
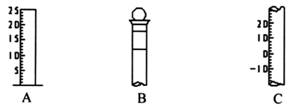
����⣺��1������������ͼ�ο�֪��AΪ��Ͳ��BΪ����ƿ��CΪ�¶ȼƣ�
��2��BΪ����ƿ������ƿ�ϱ���У��¶ȡ��̶��ߡ��ݻ�������ڢۢݣ�
��3������ƿ��ʹ��ǰ����Ҫ����Ƿ�©ˮ��
��4������480mL0.2mol/L��ϡH2SO4����ѡ��500mL����ƿ��
����ϡ�Ͷ��ɣ�ϡ��ǰ�����ʵ������䣬������Ũ����������
��Ũ��������ΪxmL
xmL��1.84g/cm3��98%=500mL��1000-3L/mL��0.2mol/L��98g/mol
��֮�ã�x��5.4
����Ӧ��ȡ��Ũ���������5.4mL��
��5��Ũ�����ܶȱ�ˮ������ˮ�ų��������ȣ�ϡ��ʱ��Ũ�������ձ��ڱ���������ˮ�У��ӱ��ò��������裻
��6��c�����ᣩ=
�������ʵ������ӻ���Һ�������Сʱ��ʵ���ܶ�ƫ��
A������Ͳ��ȡŨ����ʱ��������Ͳ�Ŀ̶ȣ�ʹ����ȡ��Ũ��������ƫ�����������Һʵ��Ũ��ƫ��
B������ƿδ���T����������Һ��������ҺŨ��û��Ӱ�죻
C��Ũ�������ձ���ϡ�ͺ�δ��ȴ������ת�Ƶ�����ƿ�У������ж��ݣ�����ƿ����Һ�¶ȸ���20��C�������������Һ���С������ƿ������ע��Һ�������ʹ��ҺŨ��ƫ�ߣ�
D��������ƿת��ʱ��������Һ�彦����ʹ���ʵ������٣������ҺŨ��ƫ�ͣ�
E���ձ�δ����ϴ�ӣ��ձ��ڱ����������ʣ�ʹ���ʵ������٣������ҺŨ��ƫ�ͣ�
F��������ƿ�ж���ʱ��������ƿ�̶��ߣ�ʹ��Һ�����С�������ҺŨ��ƫ�ߣ�
G�����ݺ�����ƿ������ҡ�ȣ����ú�Һ�治���̶��ߣ��ټ�ˮ���̶��ߣ�ʹ��Һ������������ҺŨ��ƫ�ͣ�
��ѡACF��
�ʴ�Ϊ����1����Ͳ������ƿ���¶ȼƣ�
��2���ڢۢݣ�
��3������Ƿ�©ˮ��
��4��5.4mL��500mL����ƿ��
��5����Ũ�������ձ��ڱ���������ˮ�У��ӱ��ò��������裮
��6��ACF��
��2��BΪ����ƿ������ƿ�ϱ���У��¶ȡ��̶��ߡ��ݻ�������ڢۢݣ�
��3������ƿ��ʹ��ǰ����Ҫ����Ƿ�©ˮ��
��4������480mL0.2mol/L��ϡH2SO4����ѡ��500mL����ƿ��
����ϡ�Ͷ��ɣ�ϡ��ǰ�����ʵ������䣬������Ũ����������
��Ũ��������ΪxmL
xmL��1.84g/cm3��98%=500mL��1000-3L/mL��0.2mol/L��98g/mol
��֮�ã�x��5.4
����Ӧ��ȡ��Ũ���������5.4mL��
��5��Ũ�����ܶȱ�ˮ������ˮ�ų��������ȣ�ϡ��ʱ��Ũ�������ձ��ڱ���������ˮ�У��ӱ��ò��������裻
��6��c�����ᣩ=
| n(����) |
| V |
A������Ͳ��ȡŨ����ʱ��������Ͳ�Ŀ̶ȣ�ʹ����ȡ��Ũ��������ƫ�����������Һʵ��Ũ��ƫ��
B������ƿδ���T����������Һ��������ҺŨ��û��Ӱ�죻
C��Ũ�������ձ���ϡ�ͺ�δ��ȴ������ת�Ƶ�����ƿ�У������ж��ݣ�����ƿ����Һ�¶ȸ���20��C�������������Һ���С������ƿ������ע��Һ�������ʹ��ҺŨ��ƫ�ߣ�
D��������ƿת��ʱ��������Һ�彦����ʹ���ʵ������٣������ҺŨ��ƫ�ͣ�
E���ձ�δ����ϴ�ӣ��ձ��ڱ����������ʣ�ʹ���ʵ������٣������ҺŨ��ƫ�ͣ�
F��������ƿ�ж���ʱ��������ƿ�̶��ߣ�ʹ��Һ�����С�������ҺŨ��ƫ�ߣ�
G�����ݺ�����ƿ������ҡ�ȣ����ú�Һ�治���̶��ߣ��ټ�ˮ���̶��ߣ�ʹ��Һ������������ҺŨ��ƫ�ͣ�
��ѡACF��
�ʴ�Ϊ����1����Ͳ������ƿ���¶ȼƣ�
��2���ڢۢݣ�
��3������Ƿ�©ˮ��
��4��5.4mL��500mL����ƿ��
��5����Ũ�������ձ��ڱ���������ˮ�У��ӱ��ò��������裮
��6��ACF��
���������⿼������һ�����ʵ���Ũ����Һ��Ҫ��������ƿ��ʹ����ѡ��Ũ�����ϡ�͡��������ȣ�

��ϰ��ϵ�д�
 ѧ���������ν��Ͼ���ѧ������ϵ�д�
ѧ���������ν��Ͼ���ѧ������ϵ�д� Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
�����Ŀ
��4�֣���ͨ��״���£���ͬѧȡ1 mol H2O���ȵ�100��ʱ��Һ̬ˮ������Ϊˮ��������ͼ��ʾ�����ù������� �� �仯��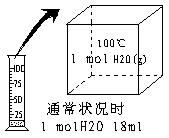
�ڱ���ѹǿ���������£�ˮ��������� ��
���������������������22.4L ��
����ͬѧ��H2��O2��ȼ�յ�ʵ�飬��ʵ��������� �� �仯���ڸñ仯�����У�һ��������ȵ��� �� ������ţ���
| A����Ӧ�������Ŀ�������������Ŀ | B����Ӧ��ԭ�������ʵ�����������ԭ�������ʵ��� |
| C����Ӧ���������������������� | D����Ӧ���������������� |
����д���������������ƣ� A �� ��B �� ��C �� ��
������B�ϱ���� �� ������ţ���
������ ���¶� �ۿ̶��� ��Ũ�� ���ݻ�
�Ǽ�������B�Ƿ�©ˮ�ķ����� ��
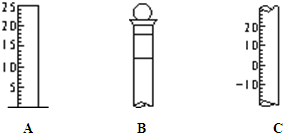 ��ͼ��ʾΪ���������IJ��ֽṹ��
��ͼ��ʾΪ���������IJ��ֽṹ��