��Ŀ����
����ʵ֤��������Ƴ�ԭ��صķ�Ӧͨ���Ƿ��ȷ�Ӧ�����л�ѧ��Ӧ�������Ͽ�����Ƴ�ԭ��ص���____________________________��
A��C(s)+H2O(g)=CO(g)+H2(g) ��H>0
B��2H2(g)+O2(g)=2H2O(1) ��H<0
C��NaOH(aq)+HC1(aq)=NaC1(aq)+H2O(1) ��H<0
����KOH��ҺΪ�������Һ��������ѡ��Ӧ���һ��ԭ��أ��������ĵ缫��ӦʽΪ_______________��
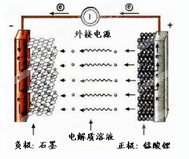
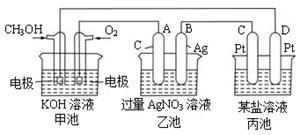
�ǵ��ԭ���ڻ�ѧ��ҵ�����Ź㷺��Ӧ�á��ֽ�����Ƶ�ԭ���ͨ����������ͼ�е�������������aΪ���Һ��X��Y��Ϊ���Ե缫����
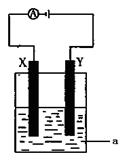
����aΪCuSO4��Һ������ʱ�Ļ�ѧ��Ӧ����ʽΪ____________________________________��
������⺬��0.04molCuSO4��0.04molNaCl�Ļ����Һ400ml������������������672 mL����״���£�ʱ����Һ��pH = �����������Һ������䣩��
A��C(s)+H2O(g)=CO(g)+H2(g) ��H>0
B��2H2(g)+O2(g)=2H2O(1) ��H<0
C��NaOH(aq)+HC1(aq)=NaC1(aq)+H2O(1) ��H<0
����KOH��ҺΪ�������Һ��������ѡ��Ӧ���һ��ԭ��أ��������ĵ缫��ӦʽΪ_______________��
�ǵ��ԭ���ڻ�ѧ��ҵ�����Ź㷺��Ӧ�á��ֽ�����Ƶ�ԭ���ͨ����������ͼ�е�������������aΪ���Һ��X��Y��Ϊ���Ե缫����

����aΪCuSO4��Һ������ʱ�Ļ�ѧ��Ӧ����ʽΪ____________________________________��
������⺬��0.04molCuSO4��0.04molNaCl�Ļ����Һ400ml������������������672 mL����״���£�ʱ����Һ��pH = �����������Һ������䣩��
��1��B
��2��O2 + 4eһ + 2 H2O = 4OHһ
��3����
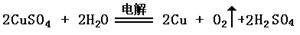
��1
��2��O2 + 4eһ + 2 H2O = 4OHһ
��3����
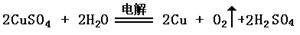
��1
�����������1��������Ŀ����������Ϣ������Ƴ�ԭ��صķ�Ӧͨ���Ƿ��ȷ�Ӧ���ų�A������Ϊ�����ԭ��صķ�Ӧ������������ԭ��Ӧ������ѡ��Bѡ���2���ڼ�����Һ��������ӦΪ��O2 + 4eһ + 2 H2O = 4OHһ����3���ٵ������ͭ��Һ�����Ļ�ѧ��Ӧ����ʽΪ��
 �ڵ��CuSO4��Һ��NaCl�����Һ�����ķ�ӦΪ��Cu2++2e- =Cu,������ӦΪ��2Cl--2e-=Cl2����������һ����672 mL��Ϊ0.03mol�����ȫ����������������������Ҫ0.06mol��������Ŀ���Ȼ���Ϊ0.04mol���������Ӳ��㣬����ṩ0.04mol�����ӣ������������0.02mol��������ʣ�µ�0.01mol����������������������������ӷŵ�������������ӷŵ硣Ҫ����0.01mol���������ݵ缫��Ӧʽ������Ҫ0.04mol�����������ӣ�������Һ����ˮ�������������Ҳ��0.04mol��Ũ��Ϊ0.1mol/L��PH=1��
�ڵ��CuSO4��Һ��NaCl�����Һ�����ķ�ӦΪ��Cu2++2e- =Cu,������ӦΪ��2Cl--2e-=Cl2����������һ����672 mL��Ϊ0.03mol�����ȫ����������������������Ҫ0.06mol��������Ŀ���Ȼ���Ϊ0.04mol���������Ӳ��㣬����ṩ0.04mol�����ӣ������������0.02mol��������ʣ�µ�0.01mol����������������������������ӷŵ�������������ӷŵ硣Ҫ����0.01mol���������ݵ缫��Ӧʽ������Ҫ0.04mol�����������ӣ�������Һ����ˮ�������������Ҳ��0.04mol��Ũ��Ϊ0.1mol/L��PH=1������������ϸ�µؿ����˵�����ԭ��أ�ÿһ��С�����漰�Ķ��ǵ�����ԭ��صij����ص�֪ʶ��ѧ����Ҫ�������գ������һ��С��PH�ļ����У��ؼ���Ҫ��������ŵ�˳���ܽ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 LiMnO4��C��
LiMnO4��C��