��Ŀ����
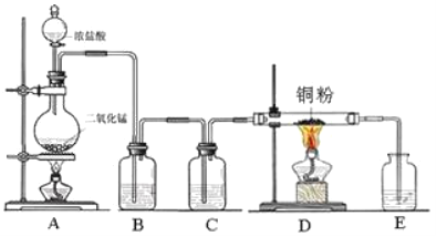
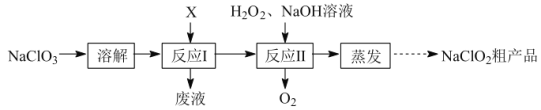
����Ŀ���������ƣ�NaClO2���Ƕ������ȣ�ClO2������Ƭ����Ҫ�ɷ֡�ʵ�����������ƣ�NaClO3��Ϊԭ�����Ƶ�ClO2�����Ʊ�NaClO2�ֲ�Ʒ����������ͼ��
��֪����ClO2�ɱ�NaOH��Һ���գ���ӦΪ2ClO2+2NaOH=NaClO3+NaClO2+H2O��
����ˮNaClO2�����ȶ�����ˮ����ʱ�����ֽ⡣
��1����Ӧ����������XΪSO2������Ʊ�ClO2��Ӧ�����ӷ���ʽΪ___��
��2��ʵ������ͼ-1��ʾ��װ���н��С�
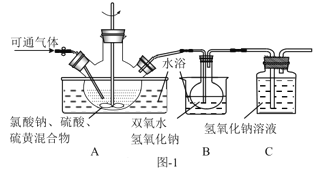
����XΪ�����Ũ���ᣬҲ�ɷ�Ӧ����ClO2���÷�Ӧ�Ͼ��ҡ����÷�Ӧ��װ��A��������ƿ�н��У��������Լ���a.Ũ���b.��ƣ�c.NaClO3��Һ��������������ƿ��˳������Ϊ___������ĸ����
�ڷ�Ӧ����˫��ˮ��������___�����ַ�Ӧʱ�䡢��Ӧ����ܼ����������䣬ʵ�������ClO2�����ʵIJ����У�װ��A�з���������ơ�___��д��һ�ּ��ɣ���
��3����װ��B����Һ����������NaClO2�������������˿��Ƶ�����Ϊ___��������ѹ��������ѹ��������ѹ������
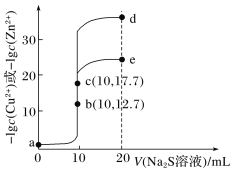
��4����Ӧ�����÷�Һ����Ҫ����ΪNa2SO4��NaHSO4��ֱ���ŷŻ���Ⱦ�������˷���Դ��Ϊ���л��â����Na2SO4��10H2O����ʯ�ࣨˮ������ƣ����벹������ʵ�鷽����___������Һ��һ���������ŷţ�ʵ������ʹ�õ��Լ����豸�У�CaO���塢��̪����ˮ�ͱ�ˮԡ������֪��CaSO4������Na2SO4ˮ��Һ��Na2SO4���ܽ��������ͼ2��ʾ��
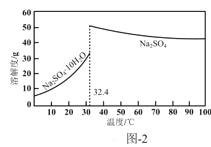
���𰸡�2ClO3-+SO2�T2ClO2+SO42- cab ����ԭ�� ˮԡ����ʱ�����¶Ȳ��ܹ��ߣ����һ��������ݵȣ� ��ѹ ���Һ�з�����������CaO���岢���裬�ñ�ˮԡ���Ʒ�Ӧ�¶ȣ�����Һȡ�����μӷ�̪������Һ��dz��ɫʱֹͣ����CaO�����ú���ˣ���ˮϴ�ӳ���2-3�εõ�ʯ�ࣻ��Һ����Ũ������ȴ�ᾧ��32.4�����£��ӽ�0�棬���ˣ����þ����ñ�ˮϴ��2-3�Σ����¸���õ�â��
��������
�ö�������NaClO3��ԭ�Ƶ�ClO2��ClO2��NaOH��Ӧ�Ƶ�NaClO3��NaClO2�����ù������⽫NaClO3��ԭ��NaClO2�Ƶ�NaClO2��Ʒ��
��1��SO2����ԭ����S���ϼ�����2����������SO42-��ClO3-��������������ԭ��ClO2��Cl���ϼ۽���1����ϵ��ӵ�ʧ�غ㡢����غ㡢ԭ���غ����д���ӷ���ʽ��2ClO3-+SO2�T2ClO2+SO42-���ʴ�Ϊ��2ClO3-+SO2�T2ClO2+SO42-��
��2���ٷ�Ӧ���ң�Ũ����ֻ����������ӣ��������ͼ��֪�ȼ�NaClO3������˳��Ϊ���ȼ�NaClO3���ټ�S������Ũ���ᣬ�ʴ�Ϊ��cab��
�ڷ�Ӧ����NaOH��ClO2��Ӧ����NaClO3��NaClO2����������������ǽ�NaClO3��ԭ��NaClO2�����������ֽ⣬�¶Ȳ���̫�ߣ����Կ���A��ˮԡ����ʱ�¶Ȳ����ߣ�������B�н��衢����һ��������ݣ��ʴ�Ϊ������ԭ����ˮԡ����ʱ�����¶Ȳ��ܹ��ߣ����һ��������ݵȣ���
��3����ˮNaClO2�����ȶ�����ˮ����ʱ�����ֽ⣬Ӧ�ü�ѹ����ˮ�־����������ʴ�Ϊ����ѹ��
��4������һ����CaOʹNaHSO4��Ӧ��CaSO4��Ϊ�˱�֤NaHSO4��Ӧ�꣬����CaO���Թ�����CaO������Һ���Լ����ˣ������÷�̪��ָʾ��������Һ��Ϊdz��ɫʱֹͣ��CaO��CaSO4������Na2SO4ˮ��Һ����ʱ���ù��ˡ�ϴ�ӵķ����õ�ʯ�࣬��ʱ��ҺΪNa2SO4��Һ��������â������ͼ�Ͽ����ɽ��¶ȵ���32.4�����£��γ�â��������â�����壬���ˡ�ϴ�ӡ�����Ϳɵ�â���ˣ�Ϊ��ʹâ�����ʽϸߣ����ñ�ˮϴ�ӣ��������ܽ���ʧ�������ʴ�Ϊ�����Һ�з�����������CaO���岢���裬�ñ�ˮԡ���Ʒ�Ӧ�¶ȣ�����Һȡ�����μӷ�̪������Һ��dz��ɫʱֹͣ����CaO�����ú���ˣ���ˮϴ�ӳ���2-3�εõ�ʯ�ࣻ��Һ����Ũ������ȴ�ᾧ��32.4�����£��ӽ�0�棬���ˣ����þ����ñ�ˮϴ��2-3�Σ����¸���õ�â����

����Ŀ��������һ����;�㷺�Ļ���ԭ�ϡ���������һ�ֹ㷺���ڵ��ӹ�ҵ��ͨѶ�������Ҫ��������Ԫ��(31Ga)��Ԫ�����ڱ���λ�ڵ�������IIIA�壬��ѧ��������Ԫ�����ơ�
��1����ͼ�ǵ���Ӧ���а�n(N2):n(H2)=1:3Ͷ�Ϻ���200����400����600���£���Ӧ�ﵽƽ��ʱ���������NH3�����ʵ���������ѹǿ�ı仯���ߡ�
��֪��N2(g)+3H2(g)![]() 2NH3(g) ��H<0
2NH3(g) ��H<0

������c��Ӧ���¶���_____��
�����ڹ�ҵ�ϳɰ��ķ�Ӧ������������ȷ����_____������ĸ����
A����ʱ�����NH3�������H2��ƽ��ת����
B��������������ԭ����һ����ø����·�����Ӧ
C����ͼ��M��N��Q��ƽ�ⳣ��K�Ĵ�С��ϵ��K(M)��K(Q)>K(N)
����ҵ�Ϻϳɰ��Ĵ���Ϊ����ý��ѡ���¶�Ϊ500����ԭ����______��
��2����ҵ������Ga��NH3�ϳɹ���뵼����ϵ�����(GaN)ͬʱ���������ɡ���Ӧ�У�����3molH2ʱ�ͻ�ų�30.8kJ��������
���÷�Ӧ���Ȼ�ѧ����ʽ��__________��
���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽ��_____��
���ں��º��ݵ��ܱ���ϵ�ڽ����������淴Ӧ�������йر�����ȷ����_____��
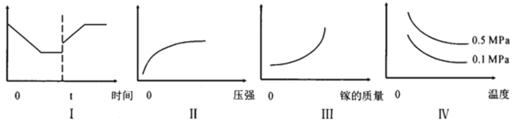
A��Iͼ�������������Ϊ����Ӧ���ʣ���tʱ�̸ı����������Ϊ����
B��IIͼ�������������Ϊ�ص�ת����
C��IIIͼ�������������Ϊ��ѧ��Ӧ����
D����ͼ�������������Ϊ��ϵ�ڻ������ƽ����Է�������
��������(GaN)�����ȶ������ܻ������ܽ����ȵ�NaOH��Һ�У��÷�Ӧ�����ӷ���ʽ��________��
��3����һ�������Ͻ���ȫ�����ռ���Һ�еõ���ҺX����֪��
Al(OH)3 | Ga(OH)3 | |
��ʽ���볣��Ka | 2��10-11 | 1��10-7 |
��ʽ���볣��Kb | 1.3��10-33 | 1.4��10-34 |
��X��Һ�л���ͨ��CO2����������������������_____��