��Ŀ����
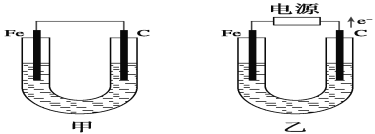
����Ŀ��ij����С����Ƶ�ʵ������ȡ����������װ����ͼ��ʾ��A�з���Ũ���ᣬB�з����Ҵ�����ˮ�����ƣ�D�з��б���̼������Һ��
��֪������ˮ�Ȼ��ƿ����Ҵ��γ�������ˮ��CaCl26C2H5OH
���й��л���ķе���ܶȣ�
�Լ� | ���� | �Ҵ� | ���� | �������� |
�е�(��) | 34.7 | 78.5 | 118 | 77.1 |
�ܶ�(g/cm3) | 0.714 | 0.789 | 1.049 | 0.903 |
��ش�
��1������A�����ƣ�______��ʵ��ǰ���װ�����������õķ�����_______________________��д����ƿB���йط�Ӧ�Ļ�ѧ����ʽ��______________________________��
��2�����θ����C��������_______________������Ӧǰ��D�м��뼸�η�̪����Һ�ʺ�ɫ����Ӧ������D�е�������_________________________________��
��3����D�з���������������г�����һ�������Ҵ������Ѻ�ˮ��Ӧ�ȼ�����ˮ�Ȼ��ƣ���ȥ______���ټ���(�˿մ�����ѡ����ѡ���������ʾ�����ˮ��)____________��Ȼ����������ռ�77�����ҵ���֣��Եõ��ϴ���������������
A��Ũ����B����ʯ��C����ˮ������D����ʯ�ң�
���𰸡���Һ©�� ���Ӻ�װ�ã��رշ�Һ©���Ļ�������˫����סԲ����ƿ���������ĩ��������ð�����ɿ��ֺ�����л���һ��Һ������װ������������ CH3COOH + HOCH2CH3 ![]() CH3COOCH2CH3 +H2O ��ֹ���� ���� ��ɫ��dz(����ʧ) �Ҵ� C
CH3COOCH2CH3 +H2O ��ֹ���� ���� ��ɫ��dz(����ʧ) �Ҵ� C
��������
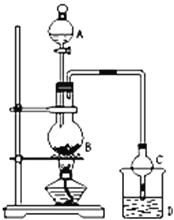
��1������A�������Ƿ�Һ©����ʵ��ǰ���װ�������Եķ����������Ӻ�װ�úƾ�������ƿ�������ܿ�������ð���������ֺ����γ�һ��ˮ���������������ã���ƿB��������Ҵ�����������Ӧ��������������ˮ����ѧ����ʽΪCH3COOH+CH3CH2OH ![]() CH3COOCH2CH3 +H2O���ʴ�Ϊ����Һ©�������Ӻ�װ�ã��رշ�Һ©���Ļ�������˫����סԲ����ƿ���������ĩ��������ð�����ɿ��ֺ�����л���һ��Һ������װ�����������ã�CH3COOH + HOCH2CH3
CH3COOCH2CH3 +H2O���ʴ�Ϊ����Һ©�������Ӻ�װ�ã��رշ�Һ©���Ļ�������˫����סԲ����ƿ���������ĩ��������ð�����ɿ��ֺ�����л���һ��Һ������װ�����������ã�CH3COOH + HOCH2CH3 ![]() CH3COOCH2CH3 +H2O��
CH3COOCH2CH3 +H2O��
��2�����θ����������Է�ֹ������ͬʱ���������ã�̼����ˮ��ʼ��ԣ��������������ڱ���̼������Һ���ܶȱ�ˮС����Һ�ֲ㣬�ϲ�Ϊ��ɫ��״Һ�壬�����̼���Ʒ�Ӧ��ʹ��Һ��ɫ��dz���ʴ�Ϊ����ֹ��������������ɫ��dz(����ʧ)��
��3���������Ϣ��֪����ˮ�Ȼ��ƿ����Ҵ��γ�������ˮ��CaCl26C2H5OH�����Ȼ��Ƶ������dz�ȥ�����Ҵ�����ˮ��������ˮ���γ������ƽᾧˮ�����ȥ�л�����������ˮ������ѡ��P2O5����ʯ�Һ�NaOH�ȹ����������Է��������������ԣ�P2O5��ˮ�����ᣩ�����������ˮ�⣬�ʴ�Ϊ���Ҵ���C��

����Ŀ��������±�ᷴӦ���Ʊ�±��������Ҫ������ʵ�����Ʊ��������1���嶡��ķ�Ӧ���£�
NaBr+H2SO4HBr+NaHSO4 ��
R��OH+HBr![]() R��Br+H2O ��
R��Br+H2O ��
���ܴ��ڵĸ���Ӧ�У�����Ũ����Ĵ�������ˮ����ϩ���ѣ�Br����Ũ��������ΪBr2�ȡ��й������б����£�
�Ҵ� | ������ | ������ | 1���嶡�� | |
�ܶ�/g��cm-3 | 0.7893 | 1.4604 | 0.8098 | 1.2758 |
�е�/�� | 78.5 | 38.4 | 117.2 | 101.6 |
��ش��������⣺
��1���������1���嶡����Ʊ�ʵ���У���������������õ�����________��������ĸ��
a��Բ����ƿ b����Ͳ c����ƿ d������©��
��2���������ˮ����______��������������������������С��������Ӧ�Ĵ�����1-�嶡��ֲ�Ʒ���ڷ�Һ©���м�ˮ�����ã�������______�������ϲ��������²����������ֲ�����
��3���Ʊ������У������Ũ����������ϡ�ͣ���Ŀ����____________��������ĸ��
a�����ٸ�����ϩ���ѵ����� b������Br2������
c������HBr�Ļӷ� d��ˮ�Ƿ�Ӧ�Ĵ���
��4������ȥ������е���������Br2���������������ʺ�����____________��������ĸ��
a��NaI b��NaOH c��NaHSO3 d��KCl
��5�����Ʊ�������ʱ�����ñ߷�Ӧ����������ķ�������������______________ ��
�����Ʊ�1���嶡��ʱȴ���ܱ߷�Ӧ�����������ԭ����_______________________��
��6���õ����������к��������Ҵ���Ϊ���Ƶô����������飬��������ˮϴ�ӣ���Һ���ټ�����ˮCaCl2����е�ʵ�������______������ĸ��
a����Һ b������ c����ȡ d������
��7��Ϊ�˼����������к�����Ԫ�أ�ͨ�����õķ�����ȡ���������飬Ȼ��______����ʵ��IJ���˳��ѡ��������ţ������� ������AgNO3 ������ϡHNO3 ������NaOH��Һ��