��Ŀ����
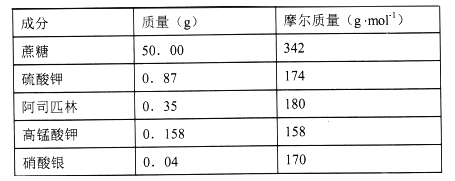
����Ŀ�������ܱ��ʼ�����һ�ֿ����ӳ����ڵ��Լ����±���500mL���ʻ����ʼ����к��еijɷ֣��Ķ���ش��������⣺
(1)���С����ܱ��ʼ����ijɷ��У����ڷǵ���ʵ���_________��
A.������� B������� C������ D.������ E��ˮ
(2)���ʻ����ʼ�����K+����˾ƥ���в���K+�������ʵ���Ũ��Ϊ__mol/L��
(3)��������500mL���ʻ����ʼ�������������У���ƽ���ձ���ҩ�ס���Ͳ����ͷ�ιܡ�_____�����ں�������д��ȱ���������ƣ�
(4)����Һ���ƹ����У����в�����ʹ���ƽ��Ũ��ƫ�͵���______��
A.����ʱ��������ƿ�̶���
B������ƿ��ʹ��ǰδ�����������������ˮ
C��ת����Һʱδϴ���ձ�
D.����ҡ�Ⱥ���Һ���������ƿ�Ŀ̶��ߣ���δ���κδ���
E����ˮʱ�����̶��ߣ��ý�ͷ�ι�����
F��δ��ȴ�����¾�ע������ƿ����
(5)�����ܱ��ʼ����и�����ص���Ҫ������___��
���𰸡�C 0.022��������500mL����ƿC��E������ϩ�����ӳ����ڡ���ɱ���ӳ��ʻ������Ⱥ������֣�
��������
���⿼������һ�����ʵ���Ũ�ȵ���Һ��
��1��������ˮ��Һ������״̬�¾����ܵ��磬���ڷǵ���ʣ�������ء�������ء���������ˮ��Һ�����Ե��磬�ʴ�ΪC����2������![]() ��֪n(K2SO4)=
��֪n(K2SO4)=![]() =0.005mol��n(KMnO4)=
=0.005mol��n(KMnO4)=![]() =0.001mol������K2SO4��2K+��֪n(K+)=2��0.005mol=0.01mol����KMnO4��K+��֪n(K+)= n(KMnO4)= 0.001mol�����ʻ����ʼ����ܵ�n(K+)=0.011mol��c(K+)=
=0.001mol������K2SO4��2K+��֪n(K+)=2��0.005mol=0.01mol����KMnO4��K+��֪n(K+)= n(KMnO4)= 0.001mol�����ʻ����ʼ����ܵ�n(K+)=0.011mol��c(K+)=![]() =0.022 mol/L����3��������500mL��Һ������ѡ��500mL����ƿ������轺ͷ�ιܶ��ݣ�����������500mL���ʻ����ʼ����������������ƽ���ձ���ҩ�ס���Ͳ����ͷ�ιܡ���������500mL����ƿ����4������ʱ�۲�Һ�温�ӣ�������Һ���ƫС������������ҺŨ��ƫ�ߣ�����ƿ��ʹ��ǰ������������ˮ����Ӱ��n��V�����Զ����ƽ��û��Ӱ�죻ת����Һ����ձ�δϴ�ӣ�����������ƿ�����ʵ�n���٣�������ҺŨ��ƫ�ͣ�����ҡ�Ⱥ���Һ���������ƿ�Ŀ̶��ߣ�һ������Һ����ƿ����ƿ��֮�䣬δ���κδ�������������ҺŨ����Ӱ�죻����ʱ����ˮ�����˿̶��ߣ�������ͷ�ι������ߵ�Һ���������������ʵ������٣�����Ũ��ƫ�ͣ�����Һע������ƿ�ᵼ������ƿ������,������,���յ�����ҺŨ�ȱ�С����ȷ��ΪC��E����5���������ֻ��һ��ǿ��������������ɱ�����ã��ӳ��ʻ�������ͬʱҲ�������ջ����ͷų�����ϩ���ӳ����ڡ�
=0.022 mol/L����3��������500mL��Һ������ѡ��500mL����ƿ������轺ͷ�ιܶ��ݣ�����������500mL���ʻ����ʼ����������������ƽ���ձ���ҩ�ס���Ͳ����ͷ�ιܡ���������500mL����ƿ����4������ʱ�۲�Һ�温�ӣ�������Һ���ƫС������������ҺŨ��ƫ�ߣ�����ƿ��ʹ��ǰ������������ˮ����Ӱ��n��V�����Զ����ƽ��û��Ӱ�죻ת����Һ����ձ�δϴ�ӣ�����������ƿ�����ʵ�n���٣�������ҺŨ��ƫ�ͣ�����ҡ�Ⱥ���Һ���������ƿ�Ŀ̶��ߣ�һ������Һ����ƿ����ƿ��֮�䣬δ���κδ�������������ҺŨ����Ӱ�죻����ʱ����ˮ�����˿̶��ߣ�������ͷ�ι������ߵ�Һ���������������ʵ������٣�����Ũ��ƫ�ͣ�����Һע������ƿ�ᵼ������ƿ������,������,���յ�����ҺŨ�ȱ�С����ȷ��ΪC��E����5���������ֻ��һ��ǿ��������������ɱ�����ã��ӳ��ʻ�������ͬʱҲ�������ջ����ͷų�����ϩ���ӳ����ڡ�

����Ŀ����֪X��Y��Z��M��R����Ԫ���У�ԭ����������������ṹ��������Ϣ���±����������Ϣ�ش��й����⣺
Ԫ�� | �ṹ��������Ϣ |
X | ԭ�ӵ�L����s����������p������ |
Y | ԭ�Ӻ����L����3��δ�ɶԵ��� |
Z | ��Ԫ�����ڱ��ĸ�Ԫ���е縺�Խ�С�ڷ� |
M | ���ʳ��¡���ѹ�������壬ԭ�ӵ�M������1��δ�ɶԵ�p���� |
R | �������ڹ���Ԫ�أ���۵��Ӳ���ܼ����ڰ����״̬ |
��1��Ԫ��M��ԭ�Ӻ����� ______ �ֲ�ͬ�˶�״̬�ĵ��ӣ�
��2������Ԫ���е�һ��������ߵ���_______дԪ�ط��ţ�
��3����Y�γɵĵ����У�![]() ����
����![]() ����Ŀ֮��Ϊ______����
����Ŀ֮��Ϊ______����![]() ��Zԭ�ӵ��ӻ���ʽΪ_____������ӵĿռ乹��Ϊ ______��
��Zԭ�ӵ��ӻ���ʽΪ_____������ӵĿռ乹��Ϊ ______��
��4��R��һ�������Ļ�ѧʽΪ![]() ����֪
����֪![]() ��ˮ��Һ���ù�����������Һ����������
��ˮ��Һ���ù�����������Һ����������![]() AgCl�������������������� ______����š�
AgCl�������������������� ______����š�
A.![]() B.
B.![]()
C.![]() D.
D.![]()