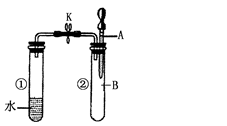
��Ŀ����
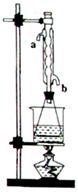
��14�֣�����ͼװ�ÿ��Խ��вⶨSO2ת����SO3��ת���ʵ�ʵ�顣��֪SO3���۵���16��8�棬�е���44��8�档��֪����װ�������漰��Ӧ�Ļ�ѧ����ʽΪ��
Na2SO3��s�� + H2SO4��98%��==Na2SO4 + H2O + SO2��

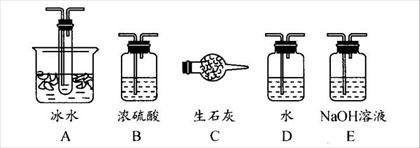
��1������ʵ����Ҫ��Ӧ���ڢ����Ӻ��ʵ�װ�á������ͼA��Eװ����ѡ�����ʺ�װ�ò����������������Ŀո��С������ӵ�װ�÷ֱ���__��__��__��
��2�����Ҵ�����ͨ��O2��ΪʹSO2�нϸߵ�ת���ʣ�ʵ�����ڼ��ȴ�����μ�Ũ�����˳���У�Ӧ��ȡ�IJ���˳���� ��
��3���״����ܵ������� ��
��4����amolNa2SO3��ĩ������Ũ������д�ʵ�飬����Ӧ����ʱ������ͨ��O2һ��ʱ����װ�â�������bg����ʵ����SO2��ת����Ϊ �����ú�a��b�Ĵ���ʽ��д��
��5��β�����θ���ܵ������� ��
Na2SO3��s�� + H2SO4��98%��==Na2SO4 + H2O + SO2��

��1������ʵ����Ҫ��Ӧ���ڢ����Ӻ��ʵ�װ�á������ͼA��Eװ����ѡ�����ʺ�װ�ò����������������Ŀո��С������ӵ�װ�÷ֱ���__��__��__��

��2�����Ҵ�����ͨ��O2��ΪʹSO2�нϸߵ�ת���ʣ�ʵ�����ڼ��ȴ�����μ�Ũ�����˳���У�Ӧ��ȡ�IJ���˳���� ��
��3���״����ܵ������� ��
��4����amolNa2SO3��ĩ������Ũ������д�ʵ�飬����Ӧ����ʱ������ͨ��O2һ��ʱ����װ�â�������bg����ʵ����SO2��ת����Ϊ �����ú�a��b�Ĵ���ʽ��д��
��5��β�����θ���ܵ������� ��
��1��B A E��B A C��3�֣�
��2���ȼ��ȴ����ٵ���Ũ���ᣨ3�֣�
��3��ƽ��ѹǿ��3�֣�
��4��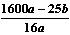 ��������
�������� ������3�֣�
������3�֣�
��5����ֹ�����е�CO2��ˮ�������ţ������2�֣�
��2���ȼ��ȴ����ٵ���Ũ���ᣨ3�֣�
��3��ƽ��ѹǿ��3�֣�
��4��
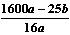 ��������
�������� ������3�֣�
������3�֣���5����ֹ�����е�CO2��ˮ�������ţ������2�֣�
��

��ϰ��ϵ�д�
 ��ͨ����ͬ����ϰ��ϵ�д�
��ͨ����ͬ����ϰ��ϵ�д� ����С����ͬ������ϵ�д�
����С����ͬ������ϵ�д�
�����Ŀ
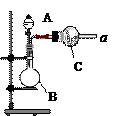


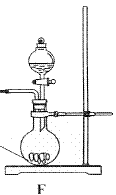
 ��Na2CO3��������A�м���ϡ���ᣬB�м���Na2CO3��NaCl�������C�м����ʯ�ҡ���װ�ô��ڽ϶�ȱ�ݣ��Ӷ�����ʵ�����ϴ�����˵�����е�����ȱ�ݣ�
��Na2CO3��������A�м���ϡ���ᣬB�м���Na2CO3��NaCl�������C�м����ʯ�ҡ���װ�ô��ڽ϶�ȱ�ݣ��Ӷ�����ʵ�����ϴ�����˵�����е�����ȱ�ݣ�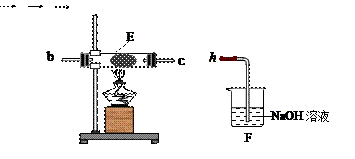 a b c h��
a b c h��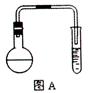
 �ζ�����̪��ָʾ�������յ�ʱ����
�ζ�����̪��ָʾ�������յ�ʱ����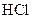 �ζ�������
�������