��Ŀ����
����Ŀ�����͵���ڷ��ٷ�չ����Ϣ�����з�����Խ��Խ��Ҫ�����á�
Li2FeSiO4�Ǽ��߷�չDZ������������ӵ�ص缫���ϣ���ƻ���ļ��������͵IJ�Ʒ���Ѿ�����һ���̶ȵ�Ӧ�á�����һ���Ʊ�Li2FeSiO4�ķ���Ϊ��
���෨��2Li2SiO3��FeSO4��Li2FeSiO4��Li2SO4��SiO2
ijѧϰС�鰴����ʵ�������Ʊ�Li2FeSiO4���ⶨ���ò�Ʒ��Li2FeSiO4�ĺ�����
ʵ�飨һ���Ʊ����̣�

ʵ�飨����Li2FeSiO4�����ⶨ��

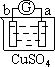
������B��ȡ20.00mL��Һ����ƿ�У���ȡ0.20molL��1������KMnO4����Һװ������C�У���������ԭ�ζ����ⶨFe2�����������ʲ�������KMnO4����Һ��Ӧ����4�εζ���ÿ������KMnO4��Һ��������£�
ʵ����� | 1 | 2 | 3 | 4 |
����KMnO4��Һ��� | 20.00mL | 19.98mL | 22.40mL | 20.02mL |
��1��ʵ�飨�����е��������ƣ�����B____������C____��
��2���Ʊ�Li2FeSiO4ʱ�����ڶ��������Χ�н��У���ԭ����____��
��3���ڲ�����ʱ�������õ��IJ��������У�������ͨ©�����ձ��⣬����____��
��4��������IJ����ǣ�__��__�����ˡ�ϴ�ӡ�
��5����ԭ��A����SO2��д���÷�Ӧ�����ӷ���ʽ____����ʱ������������ҪĿ����____��
��6���ζ��յ�ʱ����Ϊ____�����ݵζ��������ȷ����Ʒ��Li2FeSiO4����������Ϊ____�����ζ�ǰ�ζ��ܼ��촦�����ݣ��ζ�����ʧ����ʹ��õ�Li2FeSiO4����____(�ƫ�ߡ�����ƫ�͡����䡱)��
���𰸡�100mL����ƿ��ʽ�ζ��ܷ�ֹ����������������������Ũ����ȴ�ᾧSO2��2Fe3+��2H2O��2Fe2+��SO42-��4H+��ȥ������SO2������Ӱ�����Fe2+�IJⶨ��Һ��Ϊ�Ϻ�ɫ���Ұ�����ڲ���ɫ81%ƫ��
��������
ʵ��(һ)�Ʊ�����������м����ϡ������˵õ���Һ��ͨ������Ũ�������½ᾧ������ϴ�ӵõ�FeSO4![]() 7H2O�������������ȷּ���ó�������������������Li2SiO3��Ӧ�õ�Li2FeSiO4��
7H2O�������������ȷּ���ó�������������������Li2SiO3��Ӧ�õ�Li2FeSiO4��
ʵ��(��) Li2FeSiO4�����ⶨ��ȡ20.00g��Ʒ���պ��������ϡ�����ܽ���Һת�Ƶ��ձ���������������ԭ��A����SO2����ԭ������Ϊ����������������г�ȥ������SO2������Ӱ�����Fe2+�IJⶨ��ת�Ƶ�����BΪ100mL����ƿ�����Ƶõ�ȷ�����Ũ�ȵ���Һ��������B��ȡ20.00mL��Һ����ƿ������ȡ0.20molL��1������KMnO4����Һװ������C������������ԭ�ζ����ⶨFe2+��������ط�ӦΪ��MnO4-��5Fe3+��8H+��Mn2++5Fe2+��4H2O�����ʲ�������KMnO4����Һ��Ӧ���ⶨ��Һ�������ϻ�ѧ����ʽ������ϵ�������Դ˷��������
(1) ʵ���������BΪ������Һ��Ҫ������ƿ���õ���Fe2+���ӵ���Һ100mL����Ҫ����Ϊ100mL����ƿ������C��Ϊ�ζ�ʵ�����õı���Һ������ҺΪ���������Һ�����������ܸ�ʴ������Ҫʢ������ʽ�ζ�����������Ϊ��ʽ�ζ�����
��ˣ�������ȷ������100mL����ƿ����ʽ�ζ�����
��2�����෨���Ʊ�Li2FeSiO4�Ĺ��̱����ڶ��������Χ�н�������Ϊ�������Ӿ��л�ԭ�����ױ����������Զ��������Χ����Ϊ�˷�ֹ�������ӱ�������
��ˣ�������ȷ��������ֹ��������������
��3���������Ƿ���������Һ�ķ�����Ҫ���ˣ���Ϲ���װ��ѡ����������ͨ©�����ձ�����������
��ˣ�������ȷ��������������
��4������������Һ�еõ��������������ʵ�����,����Ũ��,���½ᾧ������ϴ�ӵõ���
��ˣ�������ȷ����������Ũ�������½ᾧ��
��5����ԭ��A����SO2������������л�ԭ�Ա�����������Ϊ��������Ӧ�����ӷ���ʽΪ��SO2��2Fe3+��2H2O��2Fe2+��SO42-��4H+����ʱ��������Ӧ��ȥ������SO2����Ӱ�����Fe2+�IJⶨ��
��ˣ�������ȷ������SO2��2Fe3+��2H2O��2Fe2+��SO42-��4H+����ȥ������SO2������Ӱ�����Fe2+�IJⶨ��
��6���ζ�ʵ�鷴Ӧ�յ���ж������ݸ��������Һ�������һ����Һ���Ϻ�ɫ�Ұ���Ӳ��仯��������B��ȡ20.00mL��Һ����ƿ�У���ȡ0.2000mol��L-1������KMnO4����Һװ������C�У���������ԭ�ζ����ⶨFe2+��������ط�ӦΪ��MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O�����ʲ�������KMnO4����Һ��Ӧ�����ĸ��������Һ�����3�����ϴ���ȥ������ƽ��������Һ�����������ӷ�Ӧ�Ķ�����ϵ���㣬V(��)=![]() ml=20.00ml����Ԫ���غ����
ml=20.00ml����Ԫ���غ����
MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O
5Li2FeSiO4��5Fe2+��MnO4-��
5 1
n 0.2000mol/L��0.020L
n=0.0200mol��
100ml��Һ�к����ʵ���=0.0200mol��![]() =0.1000mol��
=0.1000mol��
��Ʒ��Li2FeSiO4����������=![]() ��100%=81%��
��100%=81%��
�ζ�ǰ��ʽ�ζ��ܼ������δ�ų����ζ���������ʧ�������V(��)ƫ�����c(����)ƫ�ߣ�
��ˣ�������ȷ��������Һ��Ϊ�Ϻ�ɫ���Ұ�����ڲ���ɫ��81%��ƫ�ߡ�

����Ŀ��Ǧ��ұ���кܶ��ַ�����
��1����Ŧ�Ʒ�����Ǧ������ط�Ӧ���Ȼ�ѧ����ʽ���£�
�� 2PbS(s)��3O2(g)��2PbO(s)��2SO2(g) ��H1��a kJ��mol��1
�� PbS(s)��2PbO(s)��3Pb(s)��SO2(g) ��H2��b kJ��mol��1
�� PbS(s)��PbSO4(s)��2Pb(s)��2SO2(g) ��H3��c kJ��mol��1
��ӦPbS(s)��2O2(g)��PbSO4(s) ��H��______________ kJ��mol��1(�ú�a��b��c�Ĵ���ʽ��ʾ)��
��2����ԭ����Ǧ��������ӦPbO(s)��CO(g)![]() Pb(s)��CO2(g) ��H���÷�Ӧ��ƽ�ⳣ���Ķ���ֵ���¶ȵĹ�ϵ���±���
Pb(s)��CO2(g) ��H���÷�Ӧ��ƽ�ⳣ���Ķ���ֵ���¶ȵĹ�ϵ���±���
�¶�/�� | 300 | 727 | 1227 |
lgK | 6.17 | 2.87 | 1.24 |
�ٸ÷�Ӧ�Ħ�H_______0(ѡ�����������������)��
�ڵ�lgK��1���ں����ܱ������з���PbO��ͨ��CO����ƽ��ʱ�����������CO���������Ϊ______________ (������λ��Ч����)�����������г���һ������CO�����ƽ����_________ (�������������)�ƶ����ٴδﵽƽ��ʱ��CO��ת����_________(���������С�����䡱)��
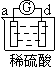
����Ŀ����a��b��c��d�ĸ������缫���йصķ�Ӧװ�ü����ַ�Ӧ�������£�
ʵ��װ�� |
|
|
|
|
����ʵ������ | a��������С b���������� | b����������� c���ޱ仯 | d���ܽ� c����������� | ������ָʾ�ڵ����� ������a������d�� |
�ɴ˿��ж������ֽ����Ļ��˳����
A a��b��c��d B b��c��d��a C d��a��b��c D a��b��d��c
����Ŀ��A��B��C��D�������ʾ�Ϊ����������ɵĿ����Ի����������������ʵ����ӣ����Ӳ����ظ���ϣ��У�
������ | Na+��Al3+��Ba2+��NH4+ |
������ | Cl����OH����CO32����SO42�� |
�ֱ�ȡ�������ʽ���ʵ�飬ʵ����������B��Һ�ֱ���C��D��ϣ����а�ɫ������������A��Һ��ε���C��Һ�У��г������ɣ������μ�A��Һʱ����������ֱ����ȫ��ʧ��A��D���ֹ��������������ɣ���������ʹʪ��ĺ�ɫʯ����Һ��������ʯī�缫���B��Һ���������ϲ���һ���д̼�����ζ������
�ش��������⣺
��1��A�����������Ӻ�C���������ӵİ뾶��С____��______�������ӷ��ţ���B��������������________
��2��C��Һ��___�ԣ�����ԡ����ԡ�������ԭ����__________________
�������ӷ���ʽ���ͣ���D�Ļ�ѧʽ��____________
��3����PtΪ�缫���1L0.1mol/LB��ˮ��Һ������·��ͨ��0.1mol����ʱ��
��Һ��pHΪ_______�����������Һ������䣩�������ĵ缫��ӦʽΪ��_____
��4����������������������ͨ��A��Һ����ǡ����ȫ��Ӧʱ������Һ�и�����
Ũ���ɴ�С������˳��Ϊ__________________________
����Ŀ����X��Y��Z��T��U���ֶ�����Ԫ�ء�X��Y��Z��Ԫ�������ڱ��е�λ����ͼ��ʾ,��Ԫ�ص�ԭ������֮����41��X��T�ĵ����ڲ�ͬ�����·�Ӧ,��������T2X(��ɫ����)��T2X2(����ɫ����)���ֻ����U������Z������ȼ��ʱ������ɫ����,�������ˮ��Һ��ʹʯ����Һ��졣
X | |
Y | Z |
(1)��Ԫ�صķ�����X__________,Y__________,Z__________,T__________,U__________
(2)Yԭ�ӵĽṹʾ��ͼΪ__________
(3)�õ���ʽ��ʾY��T��ɵĻ�������γɹ���:__________
(4) YX2��U2Y��Ӧ�Ļ�ѧ����ʽΪ_______________________,������������__________,��������Ԫ����_______��