��Ŀ����
15�� һ���¶���2L�ĺ����������У�����2moL̼��2moLCO2�������·�Ӧ��
һ���¶���2L�ĺ����������У�����2moL̼��2moLCO2�������·�Ӧ��C��s��+CO2��g��?2CO��g����H��0���������CO2�����ʵ�����ʱ��t�ı仯��ϵ��ͼ��ʾ��
��1���÷�Ӧ�ġ�S��0�����������������=�������ڽϸߣ� ��ϸߡ��ϵ͡����¶��������ڸ÷�Ӧ�Է����У�
��2����ʽ�����������¶��´˷�Ӧ��ƽ�ⳣ��K=4.0�����������һλС����
��3��������ƽ����ϵ����ͨ��CO2����CO2��ת���ʼ�С��������С�����䡢��ȷ������
��4����ͬ�¶��£�2L�ĺ����������м���4moL̼��4moLCO2��4moLCO����ʼ��Ӧʱ��v�������� v���棩���������������
��5����ͬ�¶��£�2L�ĺ����������м���4moL̼��4moLCO2���ﵽƽ�⣮����ͼ�л�����������CO2�����ʵ�����ʱ��t�仯��ϵ��Ԥ�ڽ��ʾ��ͼ����ע��ƽ��ʱCO2�����ʵ�����
���� ��1�����ݷ�Ӧǰ����������ʵ����仯���������ݡ�G=��H-T��S��0�Է������жϣ�
��2��һ���¶���2L�ĺ����������У�����2moL̼��2moLCO2������Ӧ��ƽ��ʱCO2Ϊ0.76mol��������ʽ���ƽ��ʱ�������Ũ�ȣ��ٸ���ƽ�ⳣ��K=$\frac{c{\;}^{2}��CO��}{c��CO{\;}_{2}��}$���㣻
��3���÷�Ӧ�з���Ϊ�������ķ�����ͨ��2moLCO2����ϵѹǿ����ƽ�����ƣ����˷���ת���ʣ�
��4�����ݼ����CO2��CO��Ũ�����Qc=$\frac{c{\;}^{2}��CO��}{c��CO{\;}_{2}��}$������Qc��K�Ĺ�ϵ�жϣ�
��5�������������4moL̼��4moLCO2���ﵽƽ��ʱCO2���ʵ���Ϊ2moL�����ڷ�Ӧ��Ũ������ƽ������ʱ�����̣��ݴ���ͼ��
��� �⣺��1����������ʵ���Խ������ϵ����Խ����֪C��s��+CO2��g��?2CO��g��������Ϊ�������ʵ�������ķ������Ը÷�Ӧ�ġ�S��0���֡�G=��H-T��S��0�Է������ɡ�H��0����S��0�������ڽϸ��¶��������ڸ÷�Ӧ�Է����У��ʴ�Ϊ�������ϸߣ�
��2����ͼ���֪ƽ��ʱCO2Ϊ0.76mol��
C��s��+CO2��g��?2CO��g��
��ʼ����mol����2 2 0
ת������mol����1.24 1.24 2.48
ƽ������mol����0.76 0.76 2.48
��ƽ��ʱ���������ʵ�Ũ��Ϊ��c��CO2��=$\frac{0.76mol/L}{2L}$=0.38mol/L��c��CO��=$\frac{2.48mol/L}{2L}$=1.24mol/L��
����K=$\frac{c{\;}^{2}��CO��}{c��CO{\;}_{2}��}$=$\frac{1.24{\;}^{2}}{0.38}$=4.0��
�ʴ�Ϊ��4.0��
��3���÷�Ӧ�з���Ϊ�������ķ�����ͨ��2moLCO2����ϵѹǿ������ԭƽ��Ƚϣ��൱��ƽ�����ƣ����Զ�����̼��ת���ʼ�С��
�ʴ�Ϊ����С��
��4����ͬ�¶��£�2L�ĺ����������м���4moL̼��4moLCO2��4moLCO����ʼʱc��CO2��=2mol/L��c��CO��=2mol/L��
Qc=$\frac{c{\;}^{2}��CO��}{c��CO{\;}_{2}��}$=$\frac{2{\;}^{2}}{2}$=2��K����Ӧδ�ﵽƽ�⣬��Ӧ������У�����v��������v���棩��
�ʴ�Ϊ������
��5�������������4moL̼��4moLCO2���ﵽƽ��ʱCO2���ʵ���Ϊ2moL�����ڷ�Ӧ��Ũ������ƽ������ʱ�����̣�ͼ��Ϊ��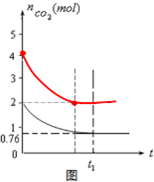 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
���� ���⿼�����ر䡢ƽ�ⳣ������ѧƽ��Ӱ�������뻯ѧƽ��ͼ��ȣ�ע����ݷ�Ӧ�������������������ƽ���Ӱ�죬�Ѷ��еȣ�

 ������ʱ����ҵ����ϵ�д�
������ʱ����ҵ����ϵ�д� ��ĩ���ƾ�ϵ�д�
��ĩ���ƾ�ϵ�д� ���ɿ��ñ���ϵ�д�
���ɿ��ñ���ϵ�д�| A�� | ����ͨ�����˵ķ��������۽����л��е��Ȼ�����Һ��ȥ | |
| B�� | ��ɢ�����ӵ�ֱ����Fe��OH��3����Һ��Fe��OH��3���壾FeCl3��Һ | |
| C�� | һ��ƽ�й������뵰������Һ��Ӳ�����Կ���һ��������ͨ· | |
| D�� | �Ʊ�Fe��OH��3����ķ����ǽ�����FeCl3��Һ�μӵ���ˮ���������Һ�ʺ��ɫ |
| A�� | �к��ȵ�ʵ������У����β����������������ͭ���棬����������к�����ֵƫС | |
| B�� | ȷ�����к��ȵ�ʵ������У�������ⶨ�¶�4�� | |
| C�� | ��ʾ�к��ȵ��Ȼ�ѧ����ʽ��H+��l��+OH-��l���TH2O��l����H=-57.3 kJ/mol | |
| D�� | ��֪2NaOH��aq��+H2SO4��aq���TNa2SO4��aq��+2H2O��l����H=-114.6 kJ/mol����÷�Ӧ���к���Ϊ114.6 kJ/mol |
 ��
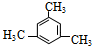
�� ���ֽṹʽ��Ϊͬ���칹�壨a��bΪ��ͬ��ԭ�ӻ�ԭ���ţ�������Ϊϩ����˳���칹�����ƶ�һ�ȱ�ϩ��ͬ���칹�壨������״�ṹ�����У�������
���ֽṹʽ��Ϊͬ���칹�壨a��bΪ��ͬ��ԭ�ӻ�ԭ���ţ�������Ϊϩ����˳���칹�����ƶ�һ�ȱ�ϩ��ͬ���칹�壨������״�ṹ�����У�������| A�� | 3�� | B�� | 4�� | C�� | 5�� | D�� | 6�� |
 �ڵ������Һ�ĵ�����װ�ã���ͼ��ʾ���У�����ijһ�������Һ����μ�����һ��Һ������������䰵��Ϩ�𣬺����������ǣ�������
�ڵ������Һ�ĵ�����װ�ã���ͼ��ʾ���У�����ijһ�������Һ����μ�����һ��Һ������������䰵��Ϩ�𣬺����������ǣ�������| A�� | ��������μ���ʳ����Һ | B�� | ��������μ���NaOH��Һ | ||
| C�� | ��������μ���Ba��OH��2��Һ | D�� | ����ͭ��Һ����μ���NaOH��Һ |
 ��
�� ��
�� ��ԭ���������������A��B��C��D��E��F��G ����Ԫ�أ�A �Ƕ������������������������ķǽ���Ԫ�أ�B Ԫ�ص�ԭ�ӼȲ���ʧȥҲ���õ����ӣ����̬ԭ����ÿ���ܼ���������ͬ��D ����������������Ӳ���֮��Ϊ3��1��E�ǵؿ��к����ڶ���ķǽ���Ԫ�أ�D��Fͬ�壻 G Ԫ��ԭ������Ϊ24��
��ԭ���������������A��B��C��D��E��F��G ����Ԫ�أ�A �Ƕ������������������������ķǽ���Ԫ�أ�B Ԫ�ص�ԭ�ӼȲ���ʧȥҲ���õ����ӣ����̬ԭ����ÿ���ܼ���������ͬ��D ����������������Ӳ���֮��Ϊ3��1��E�ǵؿ��к����ڶ���ķǽ���Ԫ�أ�D��Fͬ�壻 G Ԫ��ԭ������Ϊ24��