��Ŀ����
����Ŀ��(1)�������˻����ƻ�潫�й���ͳ�Ļ������˾����Լ��ִ��߿Ƽ���Ϊһ�塣��������ܴ����Դ�ڱ����ȼ�գ�������һ��������ȼ�ϡ��Իش��������⣺
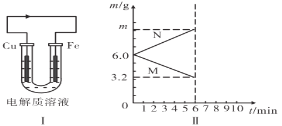
����ͼ��һ����������ȫȼ������CO2��1molH2O(l)�����е������仯ͼ������ͼ�е�������������+�������� ___��
��д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ��___��
�۶�����(CH3OCH3)��һ������ȼ�ϣ�Ӧ��ǰ��������1mol��������ȫȼ������CO2��Һ̬ˮ�ų�1455kJ��������1mol����Ͷ����ѵĻ��������ȫȼ������CO2��Һ̬ˮ���ų�1645kJ���������������У�����Ͷ����ѵ����ʵ���֮��Ϊ___��
(2)��˹������Ϊ�����ܻ�ѧ������һ����ɻ��������ɣ��������̵�����ЧӦ��ͬ�������ø�˹���ɻش��������⣺
����֪��H2O(g)�TH2O(l) ��H1=Q1kJ/mol
C2H5OH(g)�TC2H5OH(l) ��H2=Q2kJ/mol
C2H5OH(g)+3O2(g)�T2CO2(g)+3H2O(g) ��H3=Q3kJ/mol
��ʹ23gҺ̬��ˮ�ƾ���ȫȼ�գ����ָ������£������������зų�������Ϊ___kJ��
��̼(s)��������Ӧ�����ʱ������COͬʱ����������CO2�������ͨ��ʵ��ֱ�Ӳ�÷�Ӧ��C(s)+![]() O2(g)�TCO(g)����H.�������ʵ�顢���ø�˹���ɼ�����÷�Ӧ����H������ʱ��Ҫ��õ�ʵ��������___��
O2(g)�TCO(g)����H.�������ʵ�顢���ø�˹���ɼ�����÷�Ӧ����H������ʱ��Ҫ��õ�ʵ��������___��
���𰸡� C3H8(g)+5O2(g)=3CO2(g)+4H2O(l)��H=2215kJ/mol 1:3 1.5Q10.5Q2+0.5Q3 ̼��һ����̼�ı�ȼ����
��������
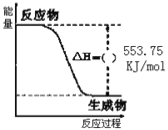
(1)����ͼ��֪����Ӧ����������������������������
����ͼ���֪��������ȫȼ������1molˮ���ʱ���H=553.75kJ/mol��
�������Ȼ�ѧ����ʽ��ϻ���������ʵ����ͷ�����ʽ����õ������Ѻͱ������ʵ���֮�ȣ�
(2)�����ݸ�˹���ɼ���ɵã�
�����ʵ�顢���ø�˹���ɼ���C(s)+ ![]() O2(g)�TCO(g)����H����Ҫ֪��̼��һ����̼��ȼ���Ȳ��ܼ���õ���
O2(g)�TCO(g)����H����Ҫ֪��̼��һ����̼��ȼ���Ȳ��ܼ���õ���
(1)����ͼ��֪����Ӧ�����������������������������÷�ӦΪ��Ӧ���ȣ���HΪ�������ʴ�Ϊ����
����ͼ���֪��������ȫȼ������1molˮ���ʱ���H=553.75kJ/mol����Ӧ���Ȼ�ѧ����ʽΪ��C3H8(g)+5O2(g)=3CO2(g)+4H2O(l)��H=2215kJ/mol���ʴ�Ϊ��C3H8(g)+5O2(g)=3CO2(g)+4H2O(l)��H=2215kJ/mol��
��1mol��������ȫȼ������CO2��Һ̬ˮ�ų�1455kJ��������1mol����Ͷ����ѵĻ��������ȫȼ������CO2��Һ̬ˮ���ų�1645kJ��������1mol��������ж��������ʵ���x���������ʵ���Ϊ1x�����Ȼ�ѧ����ʽ��֪����ȼ�շ���2215 (1x) kJ��������ɵù�ϵʽ16451455x=2215 (1x)�����x=0.75�����ϱ������ʵ���Ϊ0.25mol����������б���Ͷ��������ʵ���֮��=0.25:0.75=1:3���ʴ�Ϊ��1:3��
(2)�ٽ���֪�Ȼ�ѧ����ʽ���α��Ϊ�١��ڡ��ۣ��ɸ�˹���ɿ�֪������+����3���Ȼ�ѧ����ʽC2H5OH(l)+3O2(g)=2CO2(g)+3H2O(l) ������H1=(3Q1Q2+Q3)kJ/mol����ʹ23gҺ̬��ˮ�ƾ����ʵ���Ϊ0.5mol����ȫȼ�գ����ָ������£������������зų�������Ϊ(1.5Q10.5Q2+0.5Q3)kJ���ʴ�Ϊ��1.5Q10.5Q2+0.5Q3��
�����ʵ�顢���ø�˹���ɼ���C(s)+ ![]() O2(g)�TCO(g)����H����Ҫ֪��̼��һ����̼��ȼ���Ȳ��ܼ���õ����ʴ�Ϊ��̼��һ����̼�ı�ȼ���ȡ�
O2(g)�TCO(g)����H����Ҫ֪��̼��һ����̼��ȼ���Ȳ��ܼ���õ����ʴ�Ϊ��̼��һ����̼�ı�ȼ���ȡ�

����Ŀ����.�˳��ж����������Ը�����(��Ҫ�ɷ�Ϊ FeO �� Cr2O3������ Al2O3��SiO2 ������)Ϊ��Ҫԭ����������ԭ�Ϻ췯��(��Ҫ�ɷ� Na2Cr2O7��2H2O)��������������ͼ��
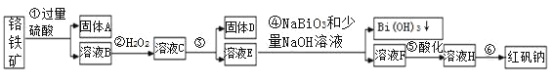
i.���£�NaBiO3������ˮ����ǿ�����ԣ����������£��ܽ�Cr3+ת��Ϊ CrO42����ii��
�������� | Fe3+ | Al3+ | Cr3+ | Fe2+ | Bi3+ |
��ʼ������pH | 2.7 | 3.4 | 5.0 | 7.5 | 0.7 |
������ȫ��pH | 3.7 | 4.9 | 5.9 | 9.7 | 4.5 |
(1)������������������Һ����ʱpHҪ����5��Ŀ����__________________��
(2)д���ܷ�Ӧ�����ӷ���ʽ____________________________________��
(3)����Һ H ��������Ũ������ȴ�ᾧ�����ˣ�ϴ�ӣ����T�ú췯�ƴ־��壬���ƺ췯�ƴ־�����Ҫ���õIJ�����______________(���������)��
��.���������������Ĺ�ҵ��ˮ�к� 5.00��10-3 mol��L-1 �� Cr2O72-���䶾�Խϴû������Ŀ�����ԱΪ�˱��Ϊ��������ˮ�����õ����Բ��� Cr0.5Fe1.5FeO4��Fe �Ļ��ϼ�����Ϊ+3��+2��������������¹������̣�
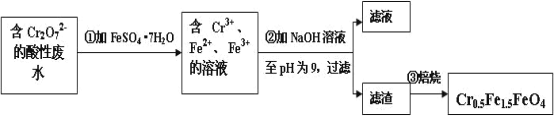
(1)�ڢٲ���Ӧ�����ӷ���ʽ��_________________________________________��
(2)��ʹ 1L �÷�ˮ�е� Cr2O72-��ȫת��Ϊ Cr0.5Fe1.5FeO4����������Ҫ����FeSO4��7H2O������Ϊ_________g (��֪ FeSO4��7H2O ��Ħ������Ϊ 278 g/mol)��
����Ŀ�����к͵ζ���ԭ����ʵ������������Ӧ�ù㷺����I2O5�ɶ����ⶨCO�ĺ������÷�Ӧԭ��Ϊ5CO+I2O5![]() 5CO2+I2����ʵ�鲽�����£�
5CO2+I2����ʵ�鲽�����£�
��ȡ250 mL����״��������CO��ij������Ʒͨ��ʢ������I2O5�ĸ��������170 ���³�ַ�Ӧ��
����ˮһ�Ҵ�Һ����ܽ����I2������100 mL��Һ��
����ȡ���������Һ25.00 mL����ƿ�У�Ȼ����0.01 mol��L-1��Na2S2O3����Һ�ζ������ı�Na2S2O3��Һ����������ʾ��
��һ�� | �ڶ��� | ������ | |
�ζ�ǰ����/mL | 2.10 | 2.50 | 1.40 |
������/mL | 22.00 | 22.50 | 21.50 |
��1�������������100 mL������Һ��Ҫ�õ��IJ����������������ձ�����Ͳ������������ͷ�ιܺ�____________________��
��2��Na2S2O3��ҺӦװ��__________������ĸ���С�

��3��ָʾ��Ӧѡ��__________���жϴﵽ�ζ��յ��������____________________________________��
��4��������Ʒ��CO���������Ϊ__________����֪��������Ʒ�������ɷֲ���I2O5��Ӧ��2Na2S2O3+I2=2NaI+Na2S4O6��
��5�����в������������CO���������ƫ�����__________������ĸ����
a���ζ��յ㸩�Ӷ���
b����ƿ�ô�����Һ��ϴ
c���ζ�ǰ�����ݣ��ζ���û������
d������100 mL������Һʱ������������
����Ŀ����֪![]() ��
��![]() ��
��![]() ��Ϊ����Ԫ�أ������������ݣ��ж�����˵����ȷ���ǣ�
��Ϊ����Ԫ�أ������������ݣ��ж�����˵����ȷ���ǣ�![]() ���� ��
���� ��
Ԫ�� |
|
|
|
���������� |
| b | a |
ԭ�Ӱ뾶/ | 0.152 | 0.143 | 0.186 |
A.![]() ��
��![]() λ��ͬһ���壬��
λ��ͬһ���壬��![]() ��
��![]() ����һ����
����һ����
B.![]() ��
��![]() λ��ͬһ���壬��
λ��ͬһ���壬��![]() ��
��![]() ����һ����
����һ����
C.![]() ��
��![]() λ��ͬһ���ڣ���
λ��ͬһ���ڣ���![]() ��ԭ������С��
��ԭ������С��![]() ��ԭ������
��ԭ������
D.![]() ��
��![]() λ��ͬһ���壬��
λ��ͬһ���壬��![]() ��ԭ������С��
��ԭ������С��![]() ��ԭ������
��ԭ������