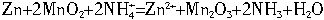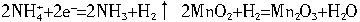��Ŀ����
п�̸ɵ�����ձ�ʹ�õĻ�ѧ��Դ�����к���MnO2��NH4Cl��ZnCl2�Ⱥ�״���пͲΪ�������ϣ�ʯīΪ�������ϣ�һ�ڸɵ�ص綯�ƺ��ڵ���ֱ�ΪW=1.5 V,r=0.25 ��,�������ʱЧ�ʦ�=75%���ɵ�صĹ���ԭ����:Zn+2MnO2+2![]() ====Zn2++Mn2O3+2NH3+H2O
====Zn2++Mn2O3+2NH3+H2O
(1)��д���ɵ�طŵ�ʱ���������缫��Ӧʽ��
������___________��������___________��
�ɵ���þ��˾ͻ��������ԭ����_________________________________��
��2��������Ӧ�У�ǰ����������������Ӧ��
2![]() +2e-====2NH3+H2��,2MnO2+H2====Mn2O3+H2O
+2e-====2NH3+H2��,2MnO2+H2====Mn2O3+H2O
���������Ӧû��MnO2�IJ��룬�ɵ�ؽ����ڳ����ȶ���������˵�����ɣ�_________
_____________________________��
��3����ͨ��10 min��ʱ���ڲμӷ�Ӧ��MnO2����ԼΪ���٣���������ĵĵ����Ƕ��٣�
��4��������ҶԷϾɵ�ؽ��л��գ��ӱ��������ͽ�Լ��Դ�ǶȽ���ΪʲôҪ���շϾɵ�ء�
��1��2MnO2+2![]() +2e-====Mn2O3+2NH3+H2O Zn-2e-====Zn2+ ��ˮ������пͲ�䱡 ��2��������Ӧ�м����H2������ʯī���棬���ӵ������ ��3��0.813 g 1.01��103 J (4)���Ի��յ������Ľ������Ϻͻ���ԭ�ϣ��Ͼɵ�����к�����̬��������������Ȼ�����¼��ѱ����⣻�Ͼ�п�̸ɵ�غ��и�Ũ�ȵ��Ȼ����Һ�����������л�ʹ�����ữ����ʹ������ˮ������
+2e-====Mn2O3+2NH3+H2O Zn-2e-====Zn2+ ��ˮ������пͲ�䱡 ��2��������Ӧ�м����H2������ʯī���棬���ӵ������ ��3��0.813 g 1.01��103 J (4)���Ի��յ������Ľ������Ϻͻ���ԭ�ϣ��Ͼɵ�����к�����̬��������������Ȼ�����¼��ѱ����⣻�Ͼ�п�̸ɵ�غ��и�Ũ�ȵ��Ȼ����Һ�����������л�ʹ�����ữ����ʹ������ˮ������
�����������⣺![]() =75%�����R=0.75 ��,I=
=75%�����R=0.75 ��,I=![]() =1.5 A;
=1.5 A;
ͨ��10 min���ĵĵ����Ϊ��Q=I��t=1.5 A��10��60 s=900 C;
ת�Ƶĵ��ӵ����ʵ���Ϊ��
![]()
�ɵ缫��Ӧʽ֪��1 mol e-��1 mol MnO2��������MnO2������Ϊ��9.34��10-3 mol��87 g��mol-1=0.813 g��
��������ĵĵ���E=I2Rt=(1.5 A)2��0.75 ����600 s=1.01��103 J��

 �����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д� �������ʱ��Ч��Ϊ?��=75%���ɵ�صĹ���ԭ���ǣ�
�������ʱ��Ч��Ϊ?��=75%���ɵ�صĹ���ԭ���ǣ�